Nuclear Magnetic Resonance (NMR) Spectroscopy
Introduction
Nuclei with an odd number of protons, neutrons, or both, will have an instrinsic
nuclear spin.
Spin quantum number for various nuclei
| Number of protons |
Number of Neutrons |
Spin Quantum Number |
Examples |
| Even |
Even |
0 |
12C, 16O, 32S |
| Odd |
Even |
1/2 |
1H, 19F, 31P |
| " |
" |
3/2 |
11B,35Cl, 79Br |
| Even |
Odd |
1/2 |
13C |
| " |
" |
3/2 |
127I |
| " |
" |
5/2 |
17O |
| Odd |
Odd |
1 |
2H, 14N |
When a nucleus with a non-zero spin is placed in a magnetic field, the
nuclear spin can align in either the same direction or in the opposite
direction as the field. These two nuclear spin alignments have different
energies and application of a magnetic field lifts the degeneracy of the
nuclear spins. A nucleus that has its spin aligned with the field will
have a lower energy than when it has its spin aligned in the opposite direction
to the field.
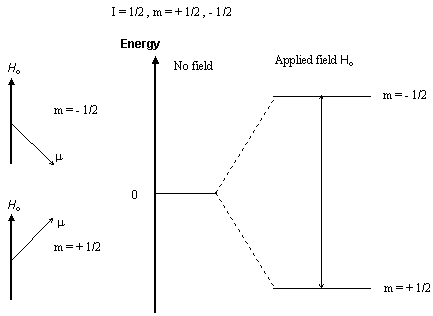
Nuclear magnetic resonance (NMR) spectroscopy is the absorption
of radiofrequency radiation
by a nucleus in a strong magnetic field. Absorption of the radiation causes
the nuclear spin to realign or flip in the higher-energy direction. After
absorbing energy the nuclei will reemit RF radiation and return to the
lower-energy state.
The energy of a NMR transition depends on the magnetic-field strength
and a proportionality factor for each nucleus called the magnetogyric ratio.
The local environment around a given nucleus in a molecule will slightly
perturb the local magnetic field exerted on that nucleus and affect its
exact transition energy. This dependence of the transition energy on the
position of a particular atom in a molecule makes NMR spectroscopy extremely
useful for determining the structure of molecules.
Instrumentation
There are two NMR spectrometer designs, continuous-wave (cw), and pulsed
or Fourier-transform (). CW-NMR spectrometers have largely been replaced
with pulsed FT-NMR instruments. However due to the lower maintenance and
operating cost of cw instruments, they are still commonly used for routine
1H NMR spectroscopy at 60 MHz. (Low-resolution cw instruments
require only water-cooled electromagnets instead of the liquid-He-cooled
superconducting magnets found in higher-field FT-NMR spectrometers.) These
two spectrometer designs are described in the following.
FT-NMR
Fourier-transform NMR spectrometers use a pulse of radiofrequency (RF)
radiation to cause nuclei in a magnetic field to flip into the higher-energy
alignment.
Due to the Heisenberg uncertainty principle, the frequency width of
the RF pulse (typically 1-10 µs) is wide enough to simultaneously
excite nuclei in all local
environments. All of the nuclei will re-emit RF radiation at their
respective resonance frequencies, creating an interference pattern in the
resulting RF emission
versus time, known as a free-induction decay (FID). The frequencies
are extracted from the FID by a Fourier transform of the time-based data.
An FT-NMR spectrometer consists of a control console, magnet, and a
coil of wire that serves as the antenna for transmitting and receiving
the RF radiation.
(Only one coil is necessary because signal reception does not begin
until after the end of the excitation pulse.) Because the FID results from
the emission due to
nuclei in all environments, each pulse contains an interference pattern
from which the complete spectrum can be obtained. Because of this multiplex
(or Fellgett)
advantage, repetitive signals can be summed and averaged to greatly
improve the signal-to-noise ratio of the resulting FID.
CW-NMR
Continuous-wave NMR spectrometers have largely been replaced with pulsed
FT-NMR instruments. However due to the lower maintenance and operating
cost
of cw instruments, they are still commonly used for routine 1H NMR
spectroscopy at 60 MHz. (Low-resolution cw instruments require only water-cooled
electromagnets instead of the liquid-He-cooled superconducting magnets
found in higher-field FT-NMR spectrometers.)
A cw-NMR spectrometer consists of a control console, magnet, and two
orthogonal coils of wire that serve as antennas for radiofrequency (RF)
radiation. One
coil is attached to an RF generator and serves as a transmitter. The
other coil is the RF pick-up coil and is attached to the detection electronics.
Since the two coils are orthogonal, the pick-up coil cannot directly recieve
any radiation from the generator coil. When a nucleus absorbs RF radiation,
it can become reoriented due to its normal movement in solution and re-emit
the RF radiation is a direction that can be recieved by the pick-up coil.
This orthogonal coil arrangement greatly increases the sensitivity of NMR
spectroscopy, similar to optical fluorescence.
Spectra are obtained by scanning the magnet and recording the pick-up
coil signal on paper at the control console.
Copyright
© 1996 by Brian M. Tissue, all rights reserved.
http://www.scimedia.com/chem-ed/spec/spin/nmr.htm, updated 9/12/96
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.

