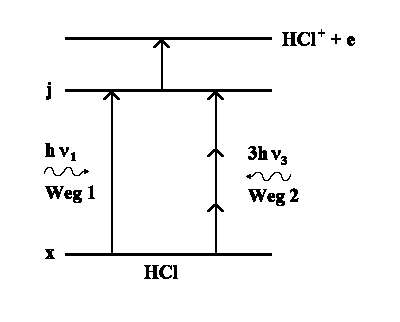
One example from chemistry:
The HCl molecule is excited by light
with the frequency n1 (1st pathway).
Simultaneously there is very intense light with frequency ν3 ,
where ν1 = 3 · ν3. Thus the HCl molecule is excited by
three photon absorptions in the same state but using pathway 2. Frequencies
ν1 and ν3 have the following particular phase ratios:
 |
(It's not important to know how to obtain it.) Finally, we
detect the HCl+-ions that are produced by HCl light excitation in the
j-state (each HCl(j) is transferred into HCl+). Now if we
measure the total ion production with definite phase ratio, then this corresponds to the
electron yield which our detector has registered in previous examples of
electron dispersion in the point x (the phase ratio corresponds to electron
dispersion here). Therefore, the phase ratio (but not the light intensity) changes but
shouldn't give rise to any ion yield changes. The phase ratio can be easily
changed whereas both light beams ( ν1 and ν3) will have
passed a long distance through an optical medium (here H2 gas) before
they both ionize HCl molecules.
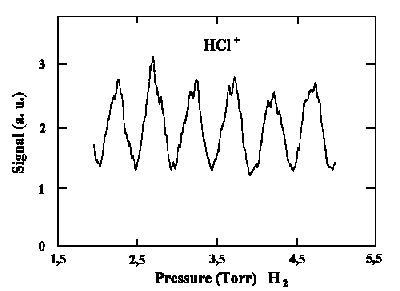 |
| Simulation of HCl+ signals when HCl is excited by two indistinguishable ways having pressure alteration of H2 as the phase ratio changes. |
Since the refractive index is n the travel time varies for different frequencies, the phase ratio alternates between v1 and v3 when the refractive index varies.
This variation of n can be reached by H2 pressure variation:
| n − 1 ~ p |
As a result we obtain the variation of HCl+ ion yield. However the intensities of both beams ν1 and ν2 stay constant! This is a purely quantum-mechanical phenomenon which is based on our knowledge of the way a molecule is excited.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.