The fundamental equations of quantum mechanics lead us to the Schroedinger equation printed below. One must produce equations which comply with the schrodinger equation and experiemental measurements. You can find one of the derivations of Schroedinger equation here.
We can write down the following differential equation for probability
amplitude when the process doesn't depend on time:
| time independent Schroedinger equation:
[−
|
where V(x) is the potential that acts upon particle t. If we substitute
p2/2m (that is directly corresponds to the
kinetic energy) for
(−h²/2m∂²/∂x²)
we follow the law of energy conservation. If we substitute E for ih∂/∂t
then it'll be the following
| time dependent Schroedinger equation:
[−
|
We can also obtain the three dimensional (x,y,z) equation, if we substitute
∂²/∂x² for ∂²/∂x²+∂²/∂y²+∂²/∂z². Erwin Schroedinger was the first scientist who
solved this equation for hydrogen atom (as a three dimensional problem). All
experimental verifications show that Schroedinger equation fully holds true in
non-relativistic quantum mechanics. Since all chemical processes run with a
speed slower than speed of light then one can say that the Schroedinger equation is a torch of truth for chemists which gives light to a dark road of
interactions between atoms and molecules. 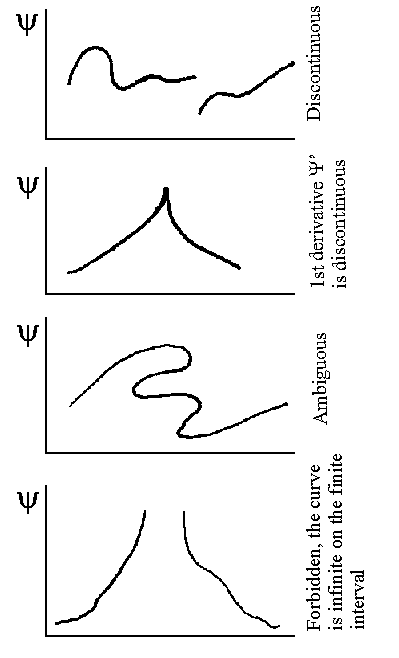 The wavefunction
y as it has been mentioned earlier has no direct
physical sense. Since the probability of detecting a particle somewhere in a space is
equal to 1, we can write the normalization condition:
The wavefunction
y as it has been mentioned earlier has no direct
physical sense. Since the probability of detecting a particle somewhere in a space is
equal to 1, we can write the normalization condition:
| -¥ò+¥|y(x,y,z)|2dx dy dz = 1 |
The expression |y(x,y,z)|2dxdydz shows the probability P(x,y,z) of detecting a particle in a position with coordinates (x,y,z) in a volume unit dV = dxdydz. P(x,y,z) unlike y(x,y,z) has direct physical meaning because the probability can be measured in experiments.
Wavefunction y requirements:
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.