 |
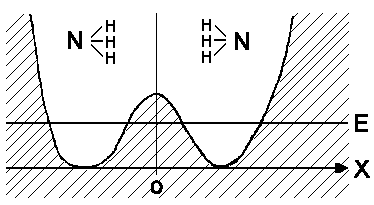
Ammonia potential V(x) for movement of N atoms:
The H atoms define the orientation of ammonia. If the nitrogen atom is situated on the right side of potential barrier there is a chance that it can overcome the barrier and move to the other side ( because N and H atoms move in opposite directions, moreover the N atom divergence is smaller than that of the H atoms).
The movement of N atom consists of left and right vibrations and a slow drift between left and right zones of the potential. The
frequency of this movement is as
follows 2,3789
· 1010 Hz (for the lowest state) This motion is consistent enough to use for time measurement! It was once the time standard for atomic clocks. Now time is measured by
the following way.
For time-independent (stationary) states the energy
levels are easily split. H atoms on the right side ''feel' the potential on
the right side. We will become acquainted with an interesting and extremely useful
description in the next chapter.
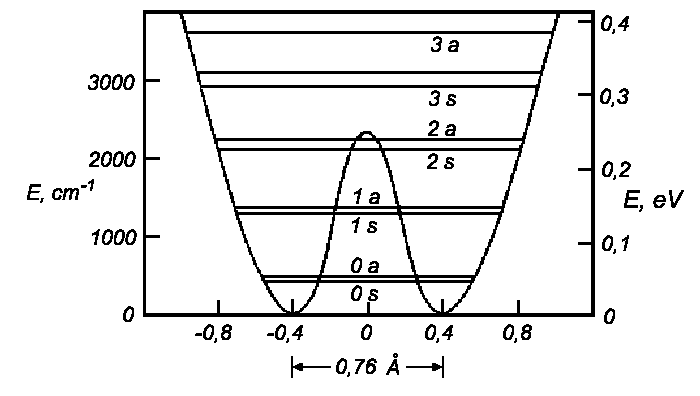 |
| Potential and energy levels for the inverse movement in NH3. |
| Level | cm−1 | eV |
| 3 a | 2681 | 0,3547 |
| 3 s | 2380 | 0,2950 |
| 2 a | 1910 | 0,2367 |
| 2 s | 1597,4 | 0,1980 |
| 1 a | 989,00 | 0,1222 |
| 1 s | 950,16 | 0,1178 |
| 0 a | 0,79 | 9,84 · 10−5 |
| 0 s | 0,00 | 0,00 |
| Vibrational levels for axial movement of N in molecule NH3 relative to the ground state. | ||
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.