Wavefunction Illustrations:
y(r,J,j) = R(r) .Y(J,j)

There are a lot of possibilities for illustrating total wavefunctions y(r,J,j) for hydrogen-like atoms. These wavefunctions consists of a radial part Rn,l(r) and and an angular part Yl,m(J,j): yn,l,m =
Rn,l(r) Yl,m(J,j).
It's quite hard to illustrate wavefunctions of this complexity in three-dimensional space, however.
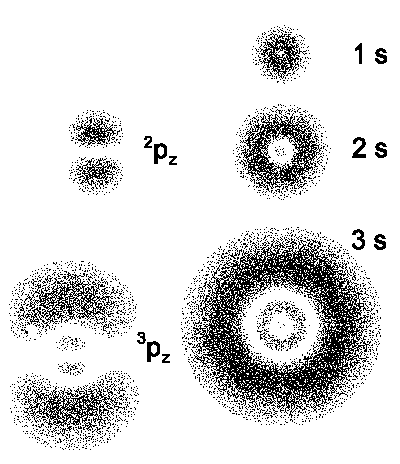
- The small illustrations below show the wavefunction y as the point density. The wavefunction of s-Electrons
(l = 0) doesn't have angular dependence and therefore it has spherical
symmetry. But it's clearly seen that there are small spherical shells in inner
regions of 2s- and 3s-Orbitals.
Radial and Angle parts:
yn,l,m =
Rn,l(r) Yl,m(J,j) |
For higher y values one will have
higher point density. |
 |
- It's also one more possibility: we plot the wavefunction's "surface" and
surface area shows wavefunction value for particular r,J,j values: y(r,J,j) = c. It's called iso-surface. If we combine such
surfaces for different r,J,j values we come to the result which is shown in the following clip for a 3dz-electron: 3dz-Isofläche:
0.82Mb
- We can cut the wavefunction into different sections, for instance, for
y-value (y = yo) we will have function y(x, y=yo, z) which can be then plotted in the
(x,z)-plane. If we combine such sections for different yo-values then we will
have a clip. One of them is shown for 3dz-electron here: 3dz-Schnitte:
0.60Mb
- The new way to illustrate the wavefunction is called "Volume rendering".
In this method, each point (r,J,j) is colored in its own
color for the whole wavefunction y(r,J,j). This illustration shows diffuse character of these
wavefunctions. The diffuseness makes it impossible to determine the direct position of
an electron. Here one can find wavefunction volume rendering for
3dz-electrons: 3dz-Rendering:
0.70Mb
Here one can also find some clips that show the
above-mentioned electron orbitals 2s
(1,0Mb), 2px(1,2Mb),
3px(1,2Mb),
3dxz(1,6Mb).
You can find more information in Internet: Orbitron.
Normalized wavefunctions of hydrogen-like atoms. For nuclear charge
Z>1 one must substitute ao with ao/Z
everywhere
| n |
l |
m |
ynlm |
Rnl(r) |
Ylm(J,j) |
| 1 |
0 |
0 |
1 s |
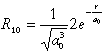 |
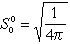 |
| 2 |
0 |
0 |
2 s |
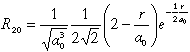 |
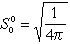 |
| 2 |
1 |
0 |
2 pz |
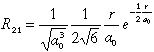 |
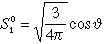 |
| 2 |
1 |
1 |
2 px |
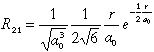 |
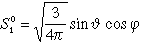 |
| 2 |
1 |
-1 |
2 py |
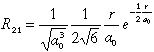 |
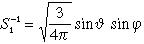 |
| 3 |
0 |
0 |
3 s |
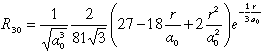 |
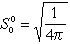 |
| 3 |
1 |
0 |
3 pz |
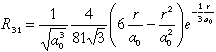 |
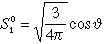 |
| 3 |
1 |
1 |
3 px |
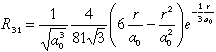 |
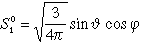 |
| 3 |
1 |
-1 |
3 py |
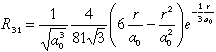 |
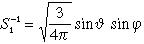 |
| 3 |
2 |
0 |
3 dz2 |
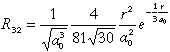 |
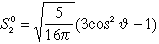 |
| 3 |
2 |
1 |
3 dxz |
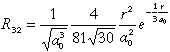 |
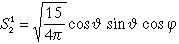 |
| 3 |
2 |
-1 |
3 dyz |
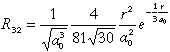 |
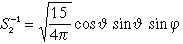 |
| 3 |
2 |
2 |
3 dx2-y2 |
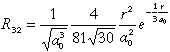 |
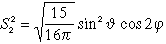 |
| 3 |
2 |
-2 |
3 dxy |
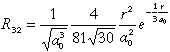 |
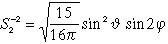 |
| To the classical illustration Radial + Angle parts: |
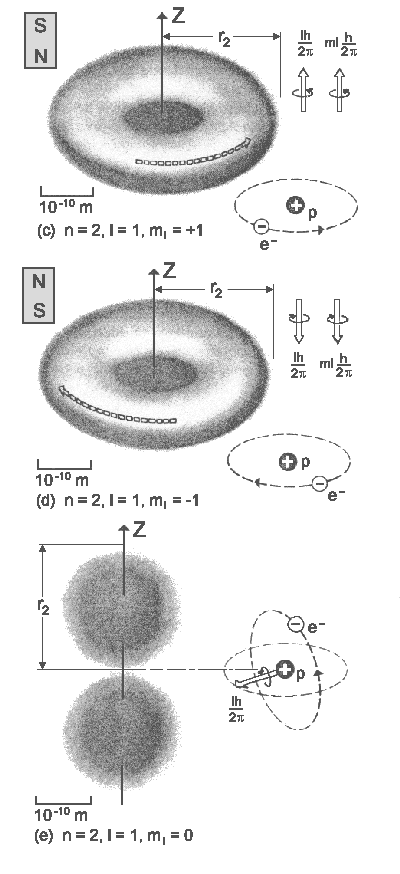 |
| The probability clouds for the first states of hydrogen atom.
The figures on the right correspond to Bohr radii. |

Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.
![]()
![]()


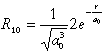
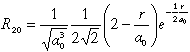
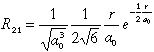
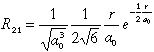
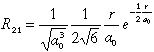
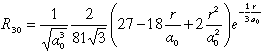
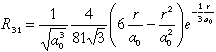
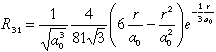
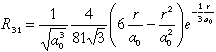
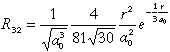
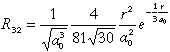
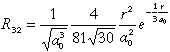
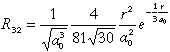
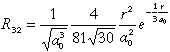

![]()