The Rotation - Energy Value ![]()
The Schroedinger equation for two particles moving in the potential which
depends only on the distance r between the particles is as follows
|
(− |
(1) |
where µ is the reduced mass of both particles A and B, mA and
mB: µ = mAmB/(mA+mB). Now
we would like to find possible solutions for y and the
corresponding energy E. Since we have the sphere-symmetrical system here
then we will use the Polar
coordinates for solving the above-mentioned equation.
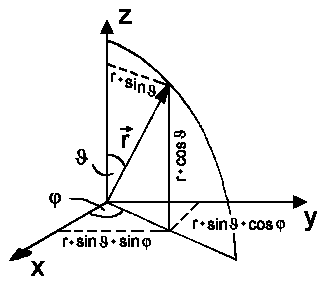 |
x = r sinJcosj
y = r sinJsinj
z = r
cosJ
We must transform only Δ into polar coordinates beacause the potential V(r) has been already given in a polar coordinate system:
Δ = ∂²/∂x² + ∂²/∂y² + ∂²/∂z²
= 1/r² ∂/∂r(r² ∂/∂r) −1/r²h²L²
where the operator L²(J,j) is the kinetic moment operator taken to the square of polar coordinates and depends only on angles, not on the distance r.
When we have the diatomic molecule A-B which rotates around its center, the bond length would change due to centrifugal forces. In our simplified treatment, we will assume the atoms to have constant distance, as if the atoms were connected by solid rod. It's also referred to as rigid rotator. The radial coordinate r is constant, the corresponding derivatives vanish and V(r) can be equal to 0. And one obtain ODE after that:
1/2µr² L²Y = E Y
where we write the wavefunction y instead of Y that can depend only on angles J,j. If we also introduce the moment of inertia I = µr² we will finally obtain:
L² Y = 2I·E Y
Therefore the ODE solution for corresponding angles corresponds to the
definition of the eigenfunction of the squared kinetic moment operator. That's
why the quantity 2I·E represents the eigenvalues. What values are possible? The
mathematical
treatment of kinetic moment eigenvalues shows that only values
l(l+1)h² are possible,
moreover l can take only integer values l = 0,1,2,3,...during
rotation:
| L² Y = |
(2) |
One can write down for the rotational energy:
| E =
|
E = B l(l+1) | l = 0,1,2,... |
where the quantity h²/2I is
shortened by B. In general B is in wavenumbers [cm-1] so that: B
[cm-1] = h²/2Ihc =
h/8π²cI where c is the speed of light.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.