The ordinary Zeeman Effect
The kinetic moment vector j and magnetic moment µj rotate together around field direction B. The additional atomic energy of atom is then as follows:
Emj = µj · B = −mj gj µB B where mj = j, j − 1, ..., −j
Here we have (2j + 1)-fold degeneracy which splits the term into 2j + 1 components. They are energy-wise equidistant.
The separation of two components with Δmj = 1 is given by:
ΔE = gj µB B
If we neglect spin and take only orbital magnetism then it will be gj = 1.
We will have the selection rule for optical transitions:
Δmj = 0, ± 1
We will have only three lines regardless of the amount of term components in
the quantum theory: the ordinary Zeeman-Triplet.
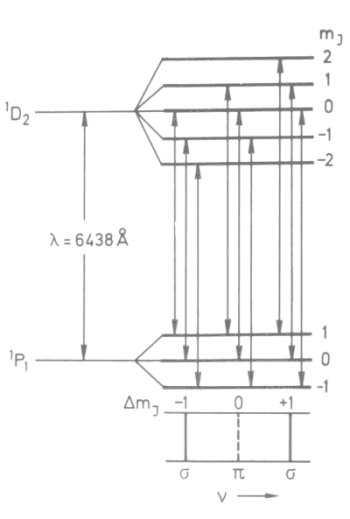 |
| Fig.
1: Ordinary Zeeman effect. The splitting of line
l
= 6438 Å of neutral Cd-Atoms, transition 1P1
- 1D2,
is then splitted into 3 components when applying magnetic field. Transitions with Δmj = 0 is called π-, transitions with Δmj = ±1 σ-transitions. The quantum number J is written using capital letter here since we're talking about many-electron atom here. |
Figure 1 shows the splitting picture for Cadmium line. The spins of both electrons are antiparallel and they compensate each other giving total spin S = 0. Transitions between components with different terms (for instance 1P1 or 1D2 in Fig.1) with the same Δmj happen since the splitting is high (we're talking about pure orbital magnetism in both cases). The unbiased line corresponds to transitions Δm = 0 and the off-center lines correspond to transitions Δm = ±1. They are circular polarized light.
If we will base upon the conservation law for total kinetic moment of
electron and light quanta together then it follows from the polarization
behavior during the Zeeman effect that the light quanta have kinetic moment 1 · h.
The inordinary Zeeman Effect
We are talking about the inordinary Zeeman effect whether the kinetic and magnetic moments of both terms (between which there will be optical transition) is described not only by quantum numbers s or l (S or L) but rather by both of them simultaneously. This is more general case.
When we have the inordinary Zeeman effect both optical terms taking place in the transition will have different g-factors. They are determined for total kinetic moments j and therefore they are referred to as gj-factors. The term splitting in the ground and excited states is quite big that's why the transition to ordinary Zeeman effect differs. The calculation of gj-factors finally gives the following:
|
|
Let's try to describe the inordinary Zeeman effect using example of NaD lines (Fig. 2).
For three terms between which there are transitions of Na atoms D-lines namely 1S½, 2P½ and 2P3/2 there are magnetic moments in the field direction
µjz = mj gj µB
And the magnetic energy of state is given again by:
Emj = − mj gj µB B
The number of splitting components in the field is given by mj and it's equal again to 2j+1. Nevertheless the separation between components with different mj - the so-called Zeeman components - isn't more for higher terms. It depends on quantum numbers l, s and j:
ΔEmj,mj−1 = gj µB B
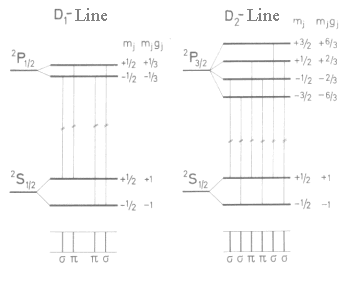 |
| Fig. 2: The inordinary Zeeman effect. The line splitting D1 and D2 of neutral Na atoms, transitions 2S½-2P½ and 2S½-2P3/2 give rise to 4 and 6 components in the magnetic field, correspondingly. |
Experiments give gj = 2 for the ground state 2S½, gj = 2/3 for the state 2P½ and 4/3 for the state 2P3/2 which were obtained by using formula for gj. The selection rule for optical transitions is given again by Δmj = 0, ± 1. Here we obtain 10 lines which are shown on fig. 2.
The meaning of Zeeman effect lies basically in the empirical term analysis. The term splitting depends unambiguously on the quantum numbers l, s and j and L, S and J for many-electron atoms, correspondingly. Therefore they can be obtained from empirical measurements of Zeeman effect.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.