The next period of the periodical table consists of 8 elements. It begins with Na then Mg which has the (3s)-shell filled. Continuing on, when moving from Al, Si, P, S, Cl, the 3p-shell is filled one electron at a time. By Ar, the 3p-shell has been completely filled. These elements have the same spectroscopic ground state terms as the above period containing Li, Be,...Ne. Their chemical behaviors are also similar. The ionization energies are slightly lower in this period (see chapter "Configuration of all elements").
It is quite amazing that the period finishes with Argon because the 3d-shell isn't fully occupied. However, the energy difference between 3p and 3d is quite large. Conversely, the energy separation between the 4s and 3d orbitals is so small that there is competition between orbitals for electrons: 4s, 4s2, 4s23d, 4s23d2, 4s23d3, 4s3d5, 4s23d5, 4s23d6, 4s23d7, 4s23d8, 4s3d10, 4s23d10. Then the 4p-shell is fully occupied and the period finishes with Krypton (z = 36).
The last consequence from z = 19 to z = 36 with occupation of 4s, 3d and 4p orbitals repeats for elements beginning from number 37 till 54. The element row with the filling of 5s, 4d and 5p orbitals is finished with Xenon.
6s-shell is occupied in the two successive elements, Cesium and Barium, and the the new 5d-orbital occupation begins then from Lanthanum. However, beginning from the next element, Cerium, the new 4f-shell begins to be occupied. The 4f-shell is fully occupied in 71st element, Lutetium. The binding energy of 4f-electrons is roughly the same as that of the 5d-electrons so that it's possible to have small variations while binding.
Beginning from 72nd element, the 5d-shell is occupied completely in Mercury. The 6p-orbital is closed in Ru (z = 86).
The next row is composed in the same way as the previous one from Z = 55
till Z = 86 (Cs - Ru); so first 7s-orbital then 6d ↔ 5f in mixture. However, since nuclei begin to be more unstable and short-lived (sphere of
nuclear physics knowledge) experimental values are no longer reliable for such
heavy atoms. We don't, however, doubt the orbital construction principle for
these atoms and were still sure that the element with Z= 118 will be a noble gas. The
possible Configurations
till Z = 112 are given on the below illustration.
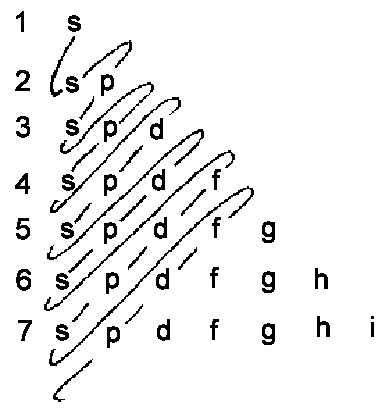 |
| The rough scheme of the order of occupied energy states is given by a curve above. |
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.