 |
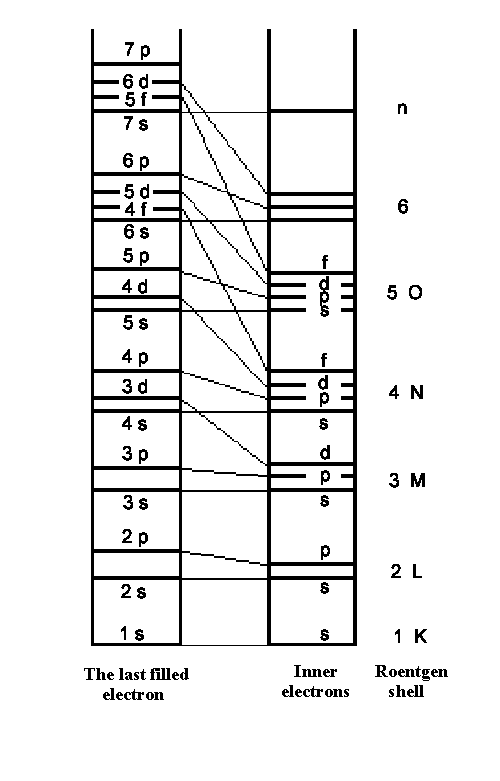
| Fig.1: The rough structure of atomic energy levels and level series for the outmost electron and inner electrons. The most stable configuration is the following: the lower energy separation overlaps with one of the higher levels. |
 |
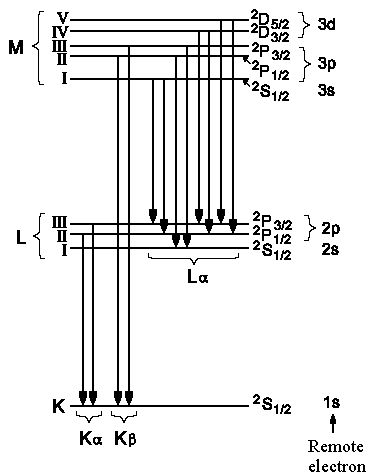 |
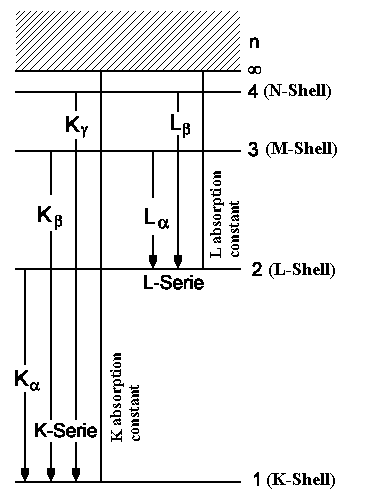
| Fig. 2: Roentgen transitions in an atom with atom number Z ~ 36. | Fig.3: Fine structure of Roentgen transitions. |
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.