Our understanding of the behavior of an electron in a hydrogen atom lets us begin study of many-electron atoms.
The whole system functions properly because internal electrons making up the closed shell
have zero total angular momentum and zero total spin. The effect of this closed
shell, together with the nuclear potential of the external valence electrons, can
be expressed by the central potential V(r). The Hamiltonian operator for the
valence electrons then is as follows (we're neglecting the nuclear spin here):
|
H = T
+ V(r) + |
Hee + | Hss + | Hsl |
|
kinetic + potential energy |
electrostatic interaction of the external electrons |
spin - spin interaction |
spin - orbital interaction |
Depending on strength of the last three terms, there are two boundary conditions:
1.)
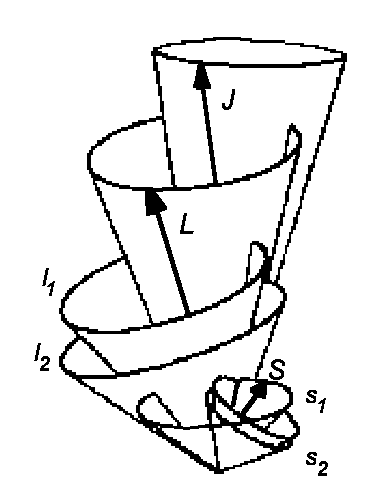
The orbital angular momenta and spins are considered independently. The
orbital anglular momentum ![]() i of single electrons are then summed up to produce the total angular momentum:
i of single electrons are then summed up to produce the total angular momentum:
![]() =
Σi
=
Σi ![]() i
i
and also the individual spins are summed up giving the total spin:
![]() =
Σi
=
Σi ![]() i
i
After that we have the weak spin-orbital interaction (Hsl) and the
moments ![]() and
and ![]() are summed
up giving the total kinetic moment:
are summed
up giving the total kinetic moment:
![]() =
= ![]() +
+ ![]()
This type of coupling is also referred to as LS-Coupling or Russel-Saunders-Coupling (1925) and it holds true almost for all atoms (all light atoms).
The quantum numbers of Russel-Saunders-Coupling are orbital moment L, spin S,
angular momentum J (nomenclature: 2S+1LJ) and the
magnetic quantum number M isn't clearly seen in this nomenclature.
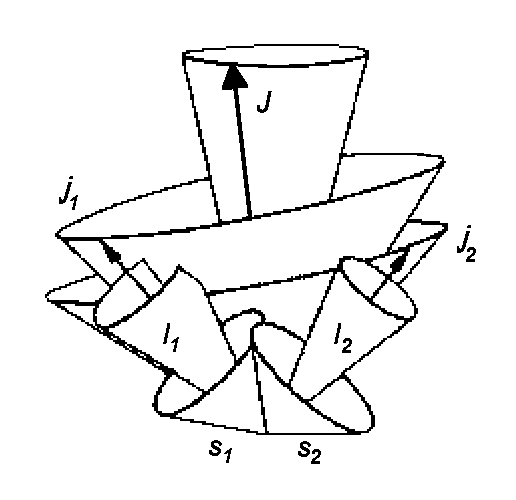
2.)
In this type of spin-orbital coupling, the interaction between spin of a
single electron and spin ![]() i + orbital moment
i + orbital moment ![]() i of each other electron
dominates giving the following total kinetic moment
i of each other electron
dominates giving the following total kinetic moment ![]() i of
i-electron:
i of
i-electron:
![]() i
=
i
= ![]() i
+
i
+ ![]() i
i
The total kinetic moment of the atomic state is then as follows:
![]() =
Σi
=
Σi ![]() i
i
This type of coupling is called the jj-Coupling and it can be seen only in heavy atoms because of the great volume of the electronic shell that the valence electrons are situated in. Therefore, the interaction between valency electrons is weak compared with the spin-orbital interaction of a single electron.
The quantum numbers are ji (nomenclature: (j1, j2, j3, ...)J)
To prevent from being misunderstood: the pure mathematical addition of
vectors ![]() i
and
i
and ![]() i gives the same result
i gives the same result ![]() ; but only addition of S
; but only addition of S![]() i and S
i and S![]() i and
then
i and
then ![]() +
+ ![]() ;
and also
;
and also ![]() i
+
i
+ ![]() i and then S
i and then S![]() i, correspondingly
should show us the dominating interaction. The total kinetic moment is the
conservative quantity for both LS- and jj-Coupling.
i, correspondingly
should show us the dominating interaction. The total kinetic moment is the
conservative quantity for both LS- and jj-Coupling.
 |
 |
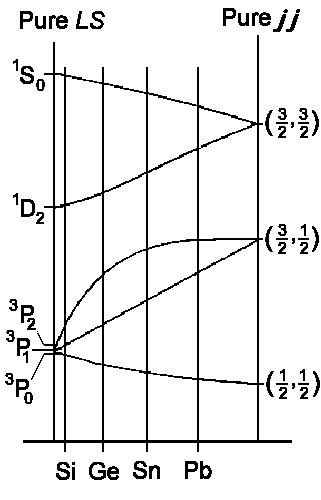 |
| jj - Coupling | Russel-Saunders- (LS-) Coupling | LS - jj Correlation for elements of the 4th main group |
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.