Discussion Fr 24.6.2005 10:30 - 11:15 am lecture hall SN 20.2
Problem Set 9 (angular momentum, rotation,
hydrogen atom)
Problem 1
Determine the angle J between the z axis and
Problem 2
a) Determine the total angular momentum J for a system
with one d electron (i.e. orbital angular momentum l = 2 and spin s =
½)? What is the degree of degeneracy?
b) What are
the possible values of the total angular momentum J if two angular momenta with j1 = 2
and j2 = 2 are combined. What is the degree of degeneracy for each
possible total angular momentum J?
Problem 3
The complete wave function y(r,J,j) for hydrogen-like atoms is given by the product of Rn,l(r) and the angular dependence Yl,m(J,j): yn,l,m(r,J,j) = Rn,l(r) Yl,m(J,j). Due to proper linear combinations of the +/-m states real wave functions are obtained. For the 3 px orbital one gets y3,1,+/-1(r,J,j) = Rn,l(r) Sl with :
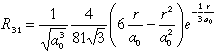
![]()
At which distance r und for what angles J and j the probability density |y3,1,+/-1(r,J,j)|² and the probability, |y3,1,+/-1(r,J,j)|² r²sinJ, of observing the electron within [r,r+dr],[J,dJ+J],[j,j+dj] reaches a maximum?
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.