| Fuel | Oxidant | Temperature (K) |
|---|---|---|
| H2 | Air | 2000-2100 |
| C2H2 | Air | 2100-2400 |
| H2 | O2 | 2600-2700 |
| C2H2 | N2O | 2600-2800 |
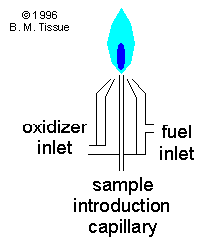 The figure shows
a total consumption burner in which the sample solution is directly aspirated
into the flame. This flame design is common for atomic emission spectroscopy.
All desolvation, atomization, and excitation occurs in the flame. Other
flame designs nebulize the sample and premix it with the fuel and oxidant
before it reaches the burner. Atomic-absorption instruments almost always
use a nebulizer and also use a slot burner to increase the path length
for the sample absorption.
The figure shows
a total consumption burner in which the sample solution is directly aspirated
into the flame. This flame design is common for atomic emission spectroscopy.
All desolvation, atomization, and excitation occurs in the flame. Other
flame designs nebulize the sample and premix it with the fuel and oxidant
before it reaches the burner. Atomic-absorption instruments almost always
use a nebulizer and also use a slot burner to increase the path length
for the sample absorption.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.