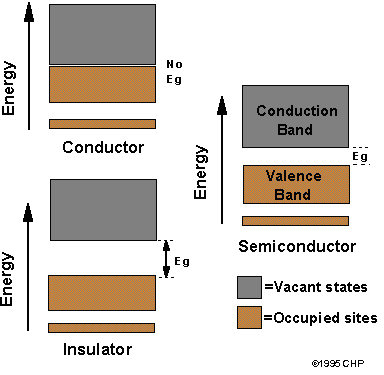
Idealized representation of energy bands and gaps

The filling of these bands and the size of the energy gap determine if a material is a conductor (a metal), a semiconductor, or an insulator. In metals there is no energy gap between filled and unfilled energy levels. A significant number of electrons are thermally excited into empty levels, creating holes in the filled band. The electrons in a conduction band and the holes in a valence band can move throughout the material, allowing it to easily conduct electricity. In semiconductors Eg is small, but large enough so that a fairly small number of electrons are in the conduction band due to thermal energy, and these materials conduct poorly. In insulators Eg is large so that electrons are not promoted to the conduction band due to thermal energy, and these materials do not conduct electricity.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.