V(R) = De (1 − 2-b(R-Re))
where De is the well depth, R is internuclear distance, Re is the equlibrium internuclear distance (bond length), and
β = pneÖ(2m/De)
νe is the vibrational constant and µ is the reduced mass.
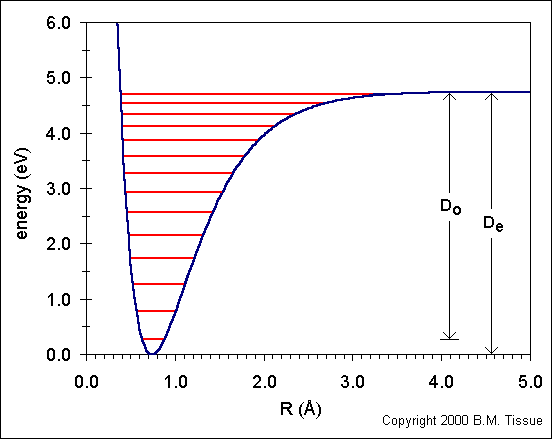
The following figure shows the ground state potential well of the H2 molecule. The curve is calculated from the Morse potential and the energy levels are calculated using the harmonic oscillator model with the first anharmonic correction.

Do is the dissociation energy, which is slightly different from the well depth, De. The vibrational energy levels in this plot are calculated from:
Ev = (v + ½) νe - (v + ½)² νexe + (v + ½)³ νeye + higher terms
where v is the vibrational quantum number, v = 0,1,2,..., and xe
and ye are the first and second anharmonicity constants, respectively.
The v = 0 level is the vibrational ground state.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.