| Number of protons | Number of Neutrons | Spin Quantum Number (I) | Examples |
|---|---|---|---|
| Even | Even | 0 | 12C, 16O, 32S |
| Odd | Even | 1/2 | 1H, 19F, 31P |
| " | " | 3/2 | 11B,35Cl, 79Br, 127I |
| Even | Odd | 1/2 | 13C |
| " | " | 5/2 | 17O |
| Odd | Odd | 1 | 2H, 14N |
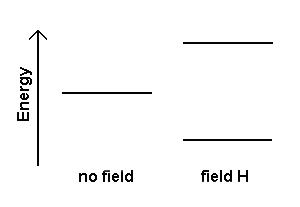
When a nucleus with a non-zero spin is placed in a magnetic field, the nuclear spin can align in either the same direction or in the opposite direction as the external magnetic field. A nucleus that has its spin aligned with the external field will have a lower energy than when it has its spin aligned in the opposite direction to the field. Thus, these two nuclear spin alignments have different energies and application of a magnetic field results in an energy level diagram like:

Nuclear magnetic resonance (NMR) spectroscopy uses the transition between these levels to detect and quantify nuclei. The magnitude of the energy splitting between these levels for nuclei in a strong magnetic field is in the range of radiofrequency (RF) radiation. Absorption of the RF radiation causes nuclear spins to realign or flip in the higher-energy direction. After absorbing energy, nuclei will reemit RF radiation and return to the lower-energy state.
The energy (and thus frequency) of an NMR transition depends on the magnetic-field strength and a proportionality factor for each nucleus called the magnetogyric ratio, γ. The frequency of a transition is given by:
ν = γ/2π H
where ν is the frequency of the resonant
radiation and H is the strength of the magnetic field.
The resonance frequencies of different nuclei in an atom are described by a relative shift compared to the frequency of a standard. This relative shift is called the chemical shift, δ, and is given by:
δ = [(νsample- nref)/νref)] 1·106
where δ has units of ppm. For 1H
NMR spectroscopy the reference compound is tetramethylsilane, Si(CH3)4,
or TMS.
For more extensive information and resources on NMR spectroscopy please
see Scott
van Bramer's NMR pages.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.