To combine successfully arbitrary atoms A and B with the LCAO approach Ψ = cAΦA + cBΦB, the wave functions
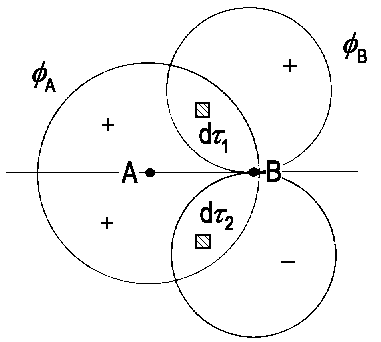 |
An example is depicted in the figure on the left, where ΦA is an s-AO and ΦB is an px-AO. Axis x is assumed to be orthogonal to the axis of the molecule AB which is in accordance with a convention saying that the molecule's axis points in direction z.
The figure illustrates that, due to contrary signs of the wave function in both lobes of the px orbital, the integral S = ∫ΦAΦBdτ = 0. Obviously, combinations of an In case the integral ∫ΦAΦBdτ is zero, the pair of wave functions ΦA and ΦB are said to be orthogonal. As shown above, different symmetry with respect to a plane that contains both nuclei causes wave functions to be orthogonal. |
| The integral ∫ΦAΦBdτ disappears due to symmetry as for any volume element dτ1 there is a respective element dτ2. The integrand delivers identical, but contrarily signed values for such pairs of volume elements. |
The following table classifies combinations of s-, p- and d-type atomic orbitals in view of the LCAO molecular orbital approach.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| # To obtain the combinations for py und dyz orbitals, substitute all indices x by indices y and vice versa in the respective row | ||
In later chapters, examples for combinations of one atomic orbital ΦA with two or more orbitals ΦB of atom B are presented. Again, possible partner orbitals are found in column "combines with ΦB". As we regard axis z as molecular axis, preferable d-orbitals are dxy,
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.