The sequence of molecular orbitals with respect to their energy is fundamental to introduce a principle for the allocation of electrons to molecular orbitals. The currently available data support the following succession of molecular orbitals:
|
|
Note that any molecular orbital of type π is doubly degenerated. For elements of the second row of the periodic table that form diatomic molecules, the σg-MO is always below the πu-MO.
The energies of MOs are often discussed with reference to a correlation diagram. It shows these energies against the internuclear distance R. For high values of R, the molecular orbitals become atomic orbitals of single atoms; for R → 0 they approach the atomic orbitals of an "united atom" that emanates from an imaginary fusion of the nuclei of both atoms. Thus, molecular orbitals are treated as a hypothetical intermediate between two extremes already described in the basic theory of atomic orbitals. The correspondence between AOs of the one and the other extreme is founded on the criteria of energy and symmetry. To find corresponding orbitals, first the AOs are listed in accordance with an increase of energy. Second, orbitals of matching symmetry are connected. The dashed lines between the energy levels indicate these correlations. Note that in such diagrams, no crossing points of lines that represent orbitals of the same symmetry exist. This reflects the non-crossing rule, of which the background is treated in detail here. Furthermore, the already familiar categories of bonding and antibonding MOs have their counterpart in the connection's slope: There are rising and falling lines. Fig.1 shows a typical correlation diagram for a homonuclear diatomic molecule.
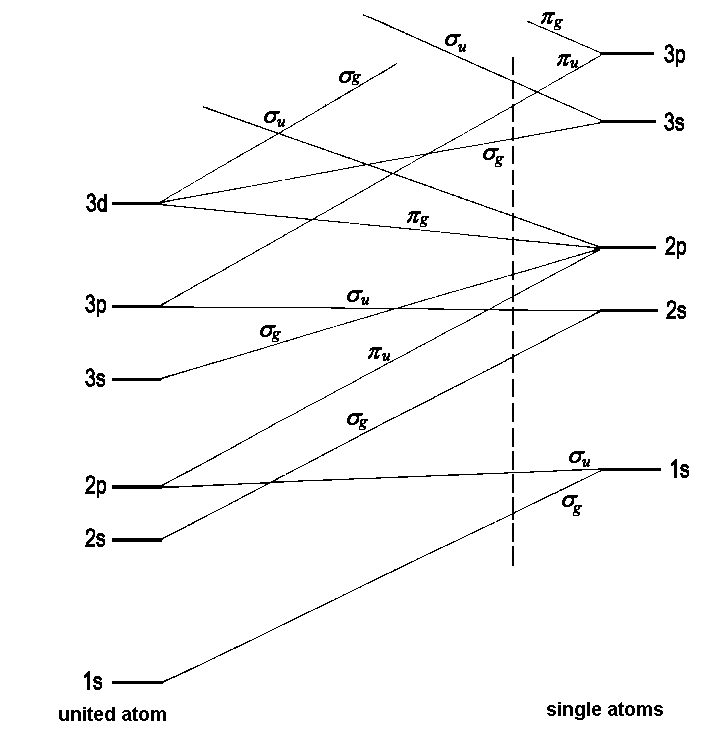 |
| Fig.1: Determination of molecular orbital energies by means of a correlation diagram. For a certain distance indicated by the vertical line, the sequence of MO energies is obtained from the diagram. |
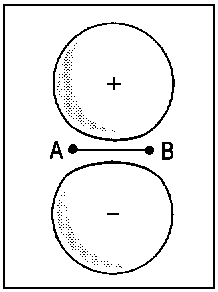
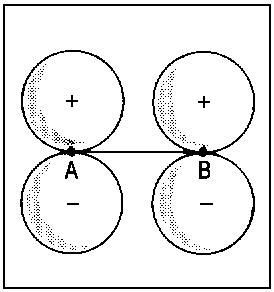
The concept of correlation is exemplified in Fig. 2 for the transition of
2px atomic orbitals of single separate atoms (right). When the nuclei A and B approach each other (R → 0), the molecular orbitals 1πu and 1πg (middle) are obtained. For R = 0, the lower extremity is reached and it is possible to describe the the former 1πu orbital as 2px and the former πg orbital as 3dzx (left).
Keep the limitations of the approach in mind. E.g. dissociation of the ionic molecule H2+ actually yields one hydrogen atom and one proton. In contrast, for
| 2px | 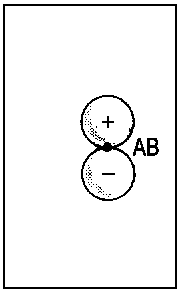 |
 |
 |
2pxA+2pxB | ||
| 3dzx | 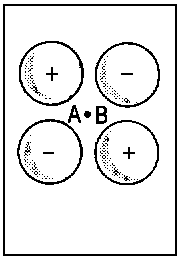 |
 |
 |
2pxA-2pxB | ||
| united nuclei | separate atoms | |||||
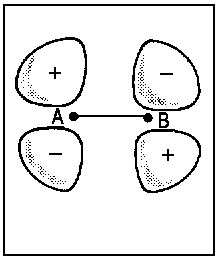
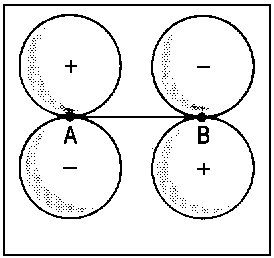
| Fig.2: Example for the correlation of orbitals for homonuclear diatomic molecules. Three internuclear distances R are shown. The upper row reveals the correspondence between the πu molecular orbital and a p-type atomic orbital of an assumed atom formed by fusion of the nuclei A and B. The lower row illustrates the same correspondence for the πg molecular orbital and an orbital of type dzx. Due to the highly charged centre of the united atom, the respective orbitals are quite compact. | ||||||
Actually, the MOs appear more complicated than a linear combination of two AOs like 2sA+2sB. For example, the 2σg and 3σg MO would be described more accurately by:
2σg : c11 (2sA
+ 2sB) + c12 (2pzA − 2pzB)
3σg : c21 (2sA
+ 2sB) + c22 (2pzA − 2pzB)
Orbitals of σu-type are to be obtained in a similar way. Take care of the symmetry of wave functions when using linear combinations of atomic orbitals. Only combinations of wave functions of identical symmetry are reasonable.
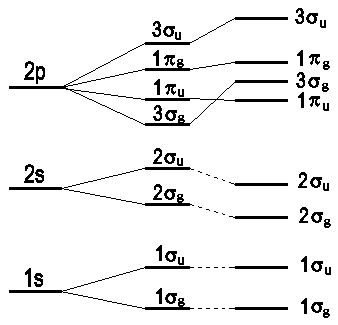 |
Fig.3: Sequence of MO levels:
|
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.