Ionisation, Photoelectron spectroscopy and REMPI
![]()
In photoelectron spectroscopy (PES), photons of high energy eject electrons of a molecule. The kinetic energy of such an eletron equals the energy hν of the hitting photon minus the ionisation energy for the electron (compare photoelectric effect). With reference to Koopmans' theorem, the one electron energy of the orbital is equated with this energy. Thus, measurements of the photoelectrons' kinetic energy allow conclusions on the energies of the orbitals of a molecule.
Applying a source that produces x-rays, the photon energy suffices to remove electrons from inner shells of atoms. Electron spectroscopy for chemical analysis (ESCA) or X-PES (or, even shorter, XPS) is an analyical technique founded on this process. As the energy of inner shell electrons is almost independent from the molecular surroundings, X-PES spectra contain information on the elements present in a sample; a feature that led to the name "... for chemical analysis". If ultraviolet radiation is used, we speak of UV-PES (or simply UPS). Here, the photon represents an energy quantum that allows the removal of electrons from a valence shell or molecular orbitals. Therefore, from UV-PES spectra, information on the energy of such orbitals is gained.
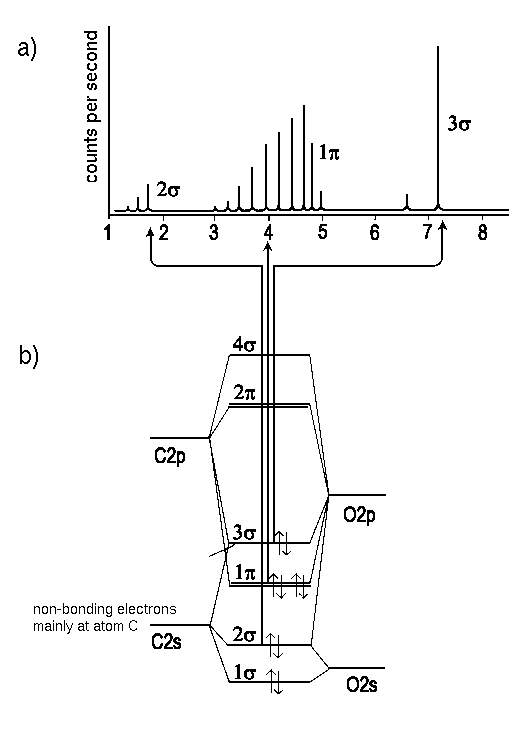 |
| Fig. 1: The UV photoelectron spectrum and the molecular orbital energy diagram of carbonmonooxide. Note that removal of an electron from the bonding 2pπ causes a series of lines that reminds us of vibrational spectra. In spectroscopy, progression is the established term for such a series which is, in fact, related to molecular vibration. In contrast, the signal due to electrons in a 2pσ-state of the CO molecule, i.e., electrons that have little effect on the intramolecular forces, have no marked fine structure. |
Removal of an electron from an valence orbital produces a cationic molecule in a nonequilibrium state, i.e. a molecules that instantly transforms potential energy in vibrational energy. Note that the kinetic energy of the photoelectron corresponds with the final vibrational state (compare Franck Condon principle) of the remaining molecule (Abb.1). Therefore, the energy distribution of photoelectrons, i.e. a spectrum recieved by photoelectron spectroscopy, displays a fine structure which can be interpreted in terms of the reached vibrational state, or, of a major or minor contribution of the removed electron to the initial molecular bonding. A long progression of lines indicates that the removed electron had been important for the molecular bonds. In contrast, electrons removed from non bonding orbitals have no pronounced effect on the position of nuclei and appear without or with inferior progression.
REMPI
The REMPI technique (Resonance enhanced multi photon ionization) uses a stepwise resonant excitation of a molecule via stable intermediate energy levels. As any species is characterized by certain energy levels, excited molecules are feasible in an almost selective way. Because it is possible to detect single ions, REMPI is extremely sensitive and a valuable method to investigate reaction dynamics. Fig. 2 depicts the principle of REMPI.
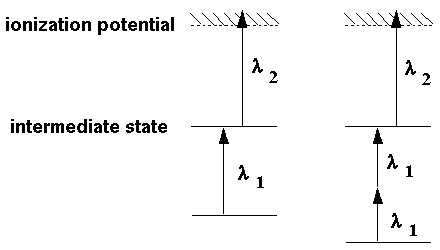 |
| Fig. 2: The principle of the REMPI technique. Left: In (1+1)-Rempi the investigated species (e.g. NO) absorbs one photon hν1, reaches an intermediate level and then absorbs another photon hν2. Right: In (2+1)-Rempi, two photons hν1 lift some species (e.g. Br) in some intermediate excited state Br* and a third photon hν2 causes ionization to Br+. |
Quite often, monochromatic laser light of an extremely high energy flux density is applied to excite the species of interest. Under such circumstances, a process that requests for n photons in a first step and m photons for final ionization has some noteworthy probability and is of analytical relevance. We speak of an (n+m)-REMPI technique, e.g. the detection of bromine based on a (2+1)-REMPI principle depicted in Fig. 2 on the right.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.