Using the MNDO approach (Modfied Neglect of Diatomic Overlap), the shape of the water molecule has been optimized and, among other characteristics, we obtaine the molecule's orbitals. This approach just deals with valence electrons, in consequence, we have eight electrons in four occupied molecular orbitals.
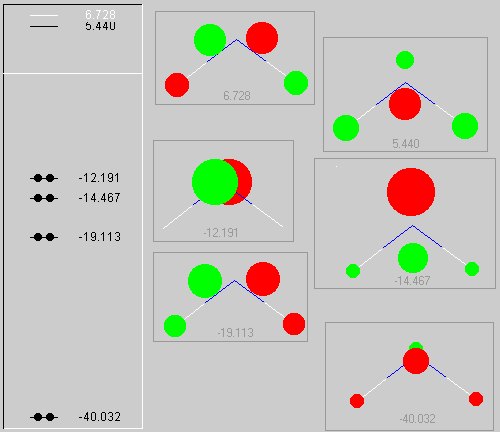
Fig. 1: The MO-Diagramm of water with a representation of the molecular orbitals.
The table on the left side contains the respective LCAO coefficients for the six atomic orbitals. The table on the right combines the energy values with the description derived from the character table for molecules of point group C2v
|
|
For the water molecule, the highest occupied orbital (HOMO) is thus the b1 orbital and he lowest unoccupied orbital (LUMO) is the 3a1.
Note
(a1)2(b2)2(a1)2(b1)2
which leads to the term symbol
1A1
This term symbol is written in majuscule letters as a multi-electron system is described here.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.