 |
| Fig. 1: Walsh-Diagram for molecules AB2 |
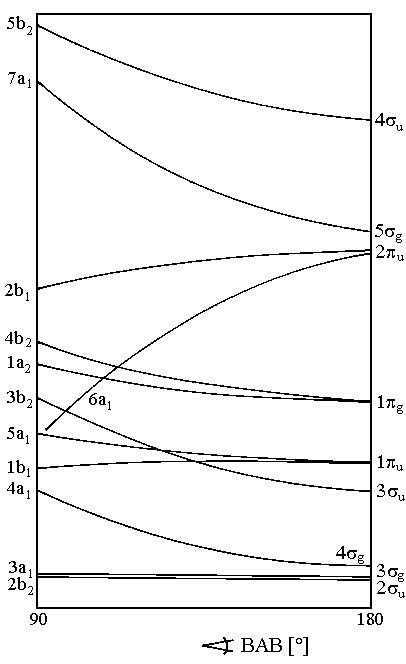
Figure 1 depicts Walsh's diagram for molecules AB2. With minor changes, it is valid for molecules ABC too. Due to the shown dependencies of the orbital energies to the angle BAB, we expect the following geometry:
| Valence electrons | Geometry |
|---|---|
| 2 - 16 | linear |
| 17 - 20 | bent |
| 21 - 24 | linear |
There is a number of molecules with 12 - 16 valence electrons known to be linear in the ground state. The table below lists some examples. Note that for molecules of point group D∞h and C∞v the same classification is used.
| Nv | Molecule | Ground state configuration |
Respective
term symbols |
|---|---|---|---|
| 12 | C3 | ...3σg2 2σu2 4σg2 3σu2 1πu4 | 1Σg+ |
| 13 | CNC, CCN | like C3 in addition 1πg | 2Πg |
| 14 | NCN, NNC | like C3 in addition 1πg2 | 3Σg−, 1Δg, 1Σg+ |
| 15 | BO2, CO2+, N3, NCO | like C3 in addition 1πg3 | 2Πg |
| 16 | CO2, N2O, N3− and many more | like C3 in addition 1πg4 | 1Σg+ |
Molecules AB2 and ABC with 16 valence electrons have a full shell configuration with with two electrons in all bonding and non-bonding orbitals whereas antibonding orbitals remain empty. These molecules are of remarkable stability. The formally non-bonding orbital 1πg seems to have a weak bonding effect.
Molecules with 17 - 20 valence electrons are less strongly bound because anti-bonding orbitals get occupied. As predicted by Walsh, these molecules are angular.
| Nv | Molecule | Ground state configuration | Term symbols |
|---|---|---|---|
| 17 | NO2, BF2 | ... 3a12 2b22 4a12 3b22 1b12 5a12 1a22 4b22 6a1 | 2A1 |
| 18 | CF2, O3, NO2− | like NO2, but 6a12 instead of 6a1 | 1A1 |
| 19 | NF2 | like CF2, but 2b12 4b2 instead of 4b22 | 2B2 |
| 20 | OF2 | like CF2, but 2b12 | 1A1 |
In accordance with Walsh's rules, the known molecules AB2 with 22 valence electrons are linear again. For instance, JF2− and XeF2
are linear and have a 1Σg+ ground state.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.