 |
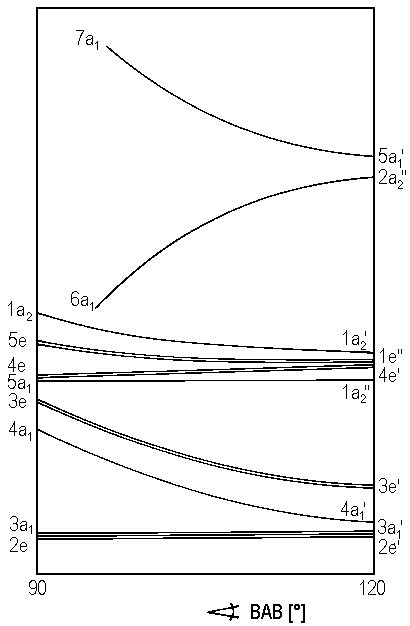
| Fig. 1: Walsh Diagram for Molecules AB3 |
Analogous to the previously presented cases it is possible to derive a Walsh diagram for molecules AB3 which is depicted in Figure 1). For these molecules, we confine ourselves to the result: The expected geometry of a molecule and the number of electrons.
| Valence electrons | Geometry |
|---|---|
| 2 - 24 | planar |
| 25 - 27 | pyramidal |
| 28 - 30 | planar |
Examples for planar molecules with 24 valence electrons are BF3, CO32−, NO3− and SO3. In contrast, molecules with 25 and 26 valence electrons, such as CF3, NF3, SO32− and PF3 have pyramidal molecules.
Though IF3 as a compound with 28 valence electrons is planar, note that it has not the threefold symmetry axis of D3h but belongs to point group C2v. This is a situation where the method suggested by Walsh appears inadequate. To solve this problem, we could use a threedimensional diagram were, in addition to the previously considered angles, an additional variable which represents the distortion of the threefold symmetry is introduced.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.