The prevalent theory at the time was that light was produced by miniature oscillating bodies. In this theory, these oscillators have an average energy of <E> = kT at a temperature T. The radiation's energy density u(ν)dν multiplied
in the spectral range [ν, ν+dν] is proportional to the number of osciallations
per volume unit multiplied by kT:
u(ν)dν = <E> dN(ν) = kT dN(ν)
| u(ν)dν = Energy of radiation in the range [ν,ν+dn]/Volume |
dN(ν) was calculated by Rayleigh and
Jeans:
dN(ν) = 8pn²/c³ dν
| u(ν) = 8pn²/c³ kT | The experiments prove the Rayleigh-Jeans law
in IR region ! |
Since u(ν) increases proportionally to ν2 , an "Ultraviolet Catastrophe" results because there would be an unreasonable amount of energy radiated with a high frequency.
1900 Max
Planck supposed that a photon cannot have any value of energy but instead only
discrete values (or quanta): An electron should have one quanta of energy since it is the most fundamental particle. The energy of an electromagnetic wave is described by E=nhν where, h is a proportionality constant. It follows that only electromagnetic radiation with an integer value for n in the above equation can be produced.
Planck derived the following formula for the spectral energy density u(ν)dν
= <E> · dN(ν) in the frequency range [ν, ν+dν] (Derivation):
| <E> | dN | |
| u(ν)dν = |
|
|
| u(ν) = 8πhν³/c³.1/ehν/kT− 1 |
This black body radiation formula perfectly coincides with the experiments.
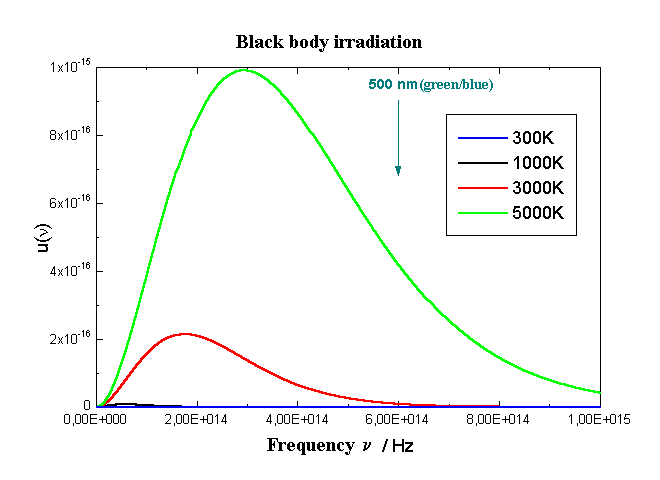
From the maximum of the distribution one can f νmax (differentiating upon ν and putting to zero) and the meaning of h can be determined from the experiment:
νmax = 2,8214 kT/h
| h = 6,626176·10-34 Js |
Integration of the Planck equation gives the
radiation law of Stefan
and Boltzmann:
|
(a = 7,56·10-16 Jm-3K-4) |
The intensity (radiated energy per surface and
time) is I = 1/4 c · a ·
T4
|
so » 5,6697 · 10-8 [Wm-2K-4] |
The radiated energy increases with the fourth power of the absolute temperature T.
Be aware that we havent yet ensured the scale accuracy of Planck constant h. Below are two problems involving the above concepts:
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.