Some number of photons are assigned to every light
wave having the frequency ν. Further, one
assumes that there are discrete atomic (or molecular) energy levels.
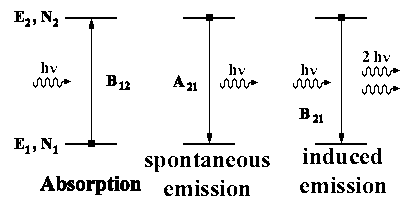 |
hν = E2 - E1 |
Many transitions occur equally in both directions (absorption and emission) in the equilibrium position; therefore the population number (?) may not have any net change:
dN2/dt = 0 (sum of the three processes)
dN2/dt = B12 u(ν)N1 − B21 u(ν)N2 − A21N2 = 0
® B12 u(ν)N1 = (B21u(ν)+A21)N2
| (B12u(ν))/(A21+B21u(ν)) | = | N2/N1 | = | e−E2/kT/e−E1/kT | = | e−hν/kT |
| → | thermal equilibrium,
thus Boltzmann distribution |
u(ν) = A21/(B12 ehν/kT − B21)
The coefficients A12, B12 and B21 are determined from experiments:
| Þ | B12 = B21 | → | u(ν) = (A21/B12)/(ehν/kT− 1) |
| A21/B12 = 8πhn ³/c³ |
The ν3 relation between spontaneous emission and absorption is
very important here !
By combining what weve learned about the
coefficients B12, B21 and A21 ,we obtain
the Planck
Radiation formula:
| u(ν) = 8πhν³/c³.1/ehν/kT− 1 |
Here we have also approached an important principle in the operation of Lasers; we must only interpret the above formula
{dN2/dt =
B12 u(ν)N1 − B21 u(ν)N2 −
A21N2} in a different
way:
Therefore we have laser operation when the photon production is
alltogether more than 0. If u(ν) increases, N2 should decrease,
i.e. dN2/dt <
0. This is important for the laser operation:
or if we neglect the spontaneous emission there will always be:
N2 > N1,
i.e. the population number (?) of the upper state should be more than the population number of the lower state. However this is not possible at thermal equilibrium position as one can see from examining the Boltzmann energy distribution. Thus lasers can be only operate in the non-equilibrium thermodynamic state.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.