Atomic-Absorption Spectroscopy (AA)
Introduction
Atomic-absorption (AA) spectroscopy uses the absorption
of light to measure the concentration of gas-phase atoms. Since samples
are usually liquids or solids, the analyte atoms or ions must be vaporized
in a flame or graphite furnace. The atoms absorb ultraviolet or visible
light and make transitions to higher electronic energy
levels. The analyte concentration is determined from the amount of
absorption. Applying the Beer-Lambert law directly
in AA spectroscopy is difficult due to variations in the atomization efficiency
from the sample matrix, and nonuniformity of concentration and path length
of analyte atoms (in graphite furnace AA). Concentration measurements are
usually determined from a working
curve after calibrating the instrument with standards of known concentration.
Schematic of an atomic-absorption experiment
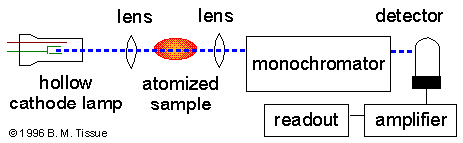
Instrumentation
Light source
The light source is usually a hollow-cathode
lamp of the element that is being measured. Lasers
are also used in research instruments. Since lasers are intense enough
to excite atoms to higher energy levels, they allow AA and atomic
fluorescence measurements in a single instrument. The disadvantage
of these narrow-band light sources is that only one element is measurable
at a time.
Atomizer
AA spectroscopy requires that the analyte atoms be in the gas phase. Ions
or atoms in a sample must undergo desolvation and vaporization in a high-temperature
source such as a flame or graphite furnace. Flame AA can only analyze solutions,
while graphite furnace AA can accept solutions, slurries, or solid samples.
Flame AA uses a slot type burner to increase the path length, and therefore
to increase the total absorbance (see Beer-Lambert
law). Sample solutions are usually aspirated with the gas flow into
a nebulizing/mixing chamber to form small droplets before entering the
flame.
The graphite furnace has several advantages over a flame. It is a much
more efficient atomizer than a flame and it can directly accept very small
absolute quantities of sample. It also provides a reducing environment
for easily oxidized elements. Samples are placed directly in the graphite
furnace and the furnace is electrically heated in several steps to dry
the sample, ash organic matter, and vaporize the analyte atoms.
Light separation and detection
AA spectrometers use monochromators and detectors for uv and visible light.
The main purpose of the monochromator is to isolate the absorption line
from background light due to interferences. Simple dedicated AA instruments
often replace the monochromator with a bandpass interference filter. Photomultiplier
tubes are the most common detectors for AA spectroscopy.
Picture of a flame atomic-absorption spectrometer

JavaScript
tour of a Flame AA Spectrometer.
Picture of a graphite-furnace atomic-absorption spectrometer

Close-up of the graphite furnace | View
of the control box
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.



