Molecular Fluorescence Spectroscopy
Introduction
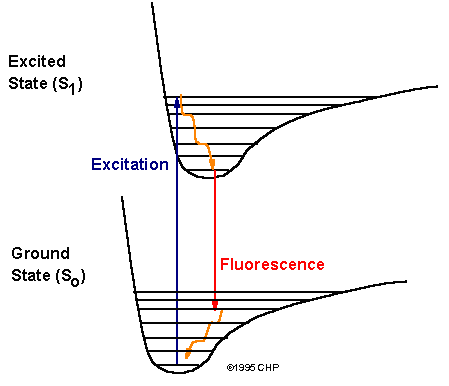
Molecular fluorescence is the optical emission
from molecules that have been excited to higher energy
levels by absorption of electromagnetic radiation.
The main advantage of fluorescence detection compared to
absorption
measurements is the greater sensitivity achievable because the fluorescence
signal has in principle a zero background. Analytical applications include
quantitative measurements of molecules in solution and fluorescence detection
in liquid chromatography. The theory of quantitative
fluorescence measurements is given in a separate document.
Transitions between molecular electronic energy levels:
Instrumentation
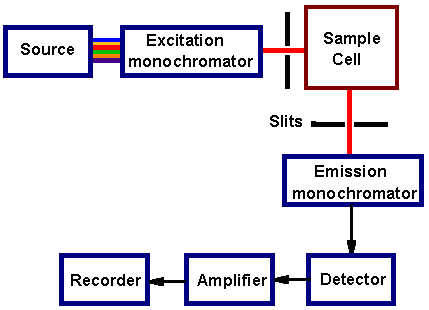
A typical fluorimeter contains an excitation
source, sample
cell, fluorescence
detector. Molecules in solution are usually excited by uv light and
the excitation source is usually a deuterium or xenon lamp. Broad-band
excitation light from a lamp passes through a monochromator,
which passes only a selected wavelength. The fluorescence is dispersed
by another monochromator and detected by a photomultiplier
tube. Scanning the excitation monochromator gives the excitation spectrum
and scanning the fluorescence monochromator gives the fluorescence spectrum.
Simple instruments sometimes use only a bandpass filter to select the excitation
wavelength.
Fluorimeter schematic
Related Topics
Because of the differences in the nature of the energy-level structure
and dynamics, discussion of atomic-fluorescence spectroscopy
(AFS) and high-resolution laser-induced molecular
fluorescence are in separate documents.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.


