 Rotation
Cn
Rotation
Cn
 Rotation
Cn
Rotation
Cn
![]()
If rotation through an angle of 360°/n about an axis of symmetry leaves the molecule in an indistinguishable condition, it is said to have an n-fold axis of symmetry. The presence of this element is denoted as Cn.
If the molecule is rotated k times through 2π/n, Cnk indicates this operation. If Cn is applied n times, the molecule is again in the initial position, i.e. Cnn≡ E.
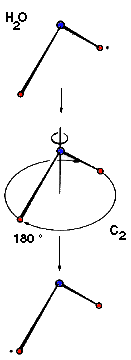
Example: Water C2 (Movie,
694kB)
 |
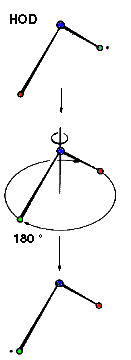 |
We can imagine the molecule H2O being turned through any angle about the bisecting line of the angle HOH, but only a rotation of 180°, (C2 ) yields an image which is indistinguishable from the original. In contrast, if the same operation is applied to HOD, the molecule displays another orientation. |
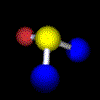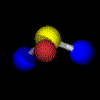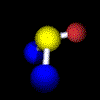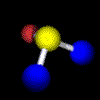
Example: Ammonia C3 (Movie, 455kB)
The trigonal pyramidal molecule NH3 possesses a three-fold rotational axis running through the centre of the non-bonding orbital, the nucleus of N and the plane spanned by the three hydrogen atoms.
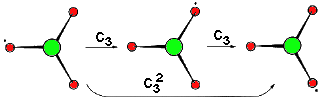 |
The rotational axis C3 is centre for two operations. One is a rotation through 120° and the other about two times this angle, the operations are denoted C3 and C32. It is not necessary to discuss a rotation by three times 120° as this operations equals identity. |
Example: Benzene (C6 , C2 )
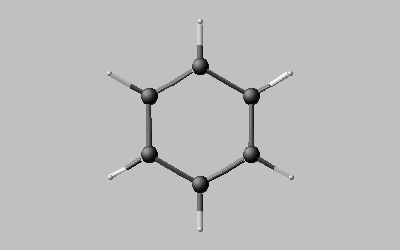 |
Benzene displays six axes C2 within the plane of the molecule and the centre of symmetry and, in addition, perpendicular to this plane, a six-fold axis C6 running as well through the centre of symmetry. The axis which produces a maximal number of identical images within one complete turn, or, in other words, with maximal n, is said to be the principal axis |
Example: Hydrogen cyanide C∞
|
In linear molecules like hydrogen cyanide, there is always an infinite-fold rotational axis C∞ which is identical to what usually is regarded as the molecules' axis. | |||||||||
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.