Cyclization of Butadiene as Example
![]()
![]()
Via cyclization, cis-Butadiene (1) is able to form Cyclobuten (2).
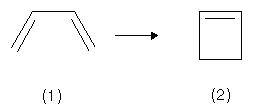
During this reaction, the pz orbitals of carbon atom 1 and 4 form one σ-bond. The remaining 2 pz orbitals of atom 2 and 3 remain present as the π bond of Cyclobuten. The figures below indicate the nodal planes for two of the four π electron systems of the butadiene molecule.
HOMO top view |
LUMO top view |
HOMO front view |
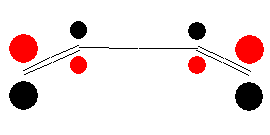
LUMO front view |
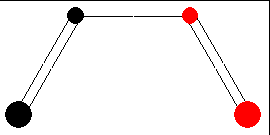
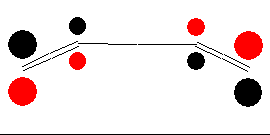
In the ground state, two electrons occupy the HOMO. The new σ-bond is formed with electrons of this orbital. To introduce bonding interaction, orbital lobes of identical signs need to overlap. Therefore, we imagine the terminal carbon atoms to rotate in one direction (both clockwise or both anti-clockwise). The figure below illustrates this so-called conrotatory mechanism of cyclization.
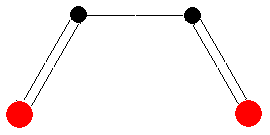
If we assume a disrotatory mechanism, no bonding interaction would occur for the HOMO. In contrast, for a molecule in an excited state, the orbital denoted as LUMO needs to be considered too. For a molecule of this state, a rotation of the terminal carbon atoms in opposite directions would allow the orbital lobes to merge. This so-called disrotatory cyclization is shown in the figure below.
The relation between the orbital of reactant and product with respect to energy and symmetry is shown in correlation diagrams.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.