The Rules of Woodward and Hoffmann
In a series of publications starting in 1965, the American chemists Robert Woodward and Roald Hoffmann suggested a theory to predict the products obtained in certain concerted reactions. Their contributions became known as Woodward-Hoffmann rules which are applied especially to pericyclic reactions which include the reorganization of electron pairs within a chain of atomic orbitals. Here, the symmetry of the involved orbitals is of fundamental importance. An adiabatic path within the correlation diagram depicts the proceeding reaction. This path connects the orbital energies of reactants and products.
For instance, we present the cyclization of the reactant (1) cis-butadiene with four π-electrons towards the product (2) cyclobutene with two π- and two σ-electrons.
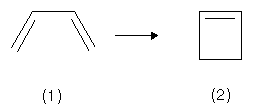
There are the cases of a conrotatory and a disrotatory mechanism. The first mechanism is characterized by terminal groups (CDH in the case of butadiene labelled with deuterium) rotating in the same, the latter by rotation in different directions.
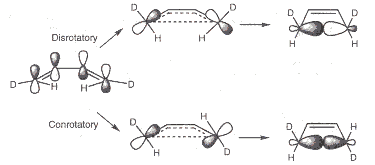
During the cyclization of a molecule which initially has one axis of rotation C2 and the mirror planes σ and σv, it is possible that the number of symmetry elements is reduced.
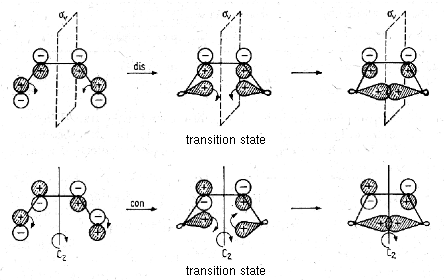
The symmetry relations between orbitals of the initial molecule and the cyclic product are illustrated below. The left scheme refers to the symmetry element C2 relevant for a conrotatory mechanism, the right scheme refers (A for antisymmetric, S for symmetric) to the symmetry element σv relevant for a disrotatory mechanism.
| Symmetry relations between four orbitals of the initial molecule butadiene and the product cyclobutene | |
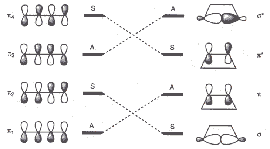 |
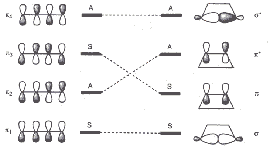 |
| for a conrotatory cyclization with C2 | for a disrotatory cyclization with σv |
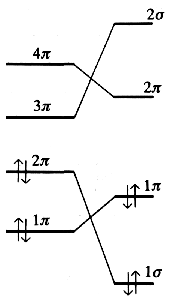
The orbital correlation diagram below reveals the energy of the four electrons involved. If the butadiene molecule performs the cyclization via a conrotary mechanism, the electrons are transferred towards a 1σ and 1π state, both orbitals are of low energy. In contrast, the disrotatory mechanism would force one pair of electrons into an antibonding 2π state of the cyclobuten molecule.
| Correlation diagram | |
 |
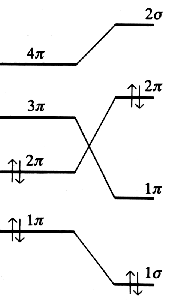 |
| conrotatory cyclization | disrotatory cyclization |
Therefore, for reaction conditions with only small amounts of available energy, we would expect a conrotatory mechanism. With stereochemistry as the point of view, the relation between reactants and products of this cyclization is well defined. This is the very heart of the rules by Woodward and Hoffmann.
In contrast, the mechanism of a cyclization introduced by the energy of a photon involves an excited state with an electronic configuration
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.