The structure of periodical system of elements is essentially described by the Pauli extraction principle (Wolfgang Pauli) which we have already discussed earlier. It sounds in the following way as applied to an atom:
"Electrons in an atom must be represented by different sets of quantum numbers (n, l, ml, ms)."
This rule is a particular case of more general statement:
| The total wavefunction of electron system must be antisymmetrical. |
We would like to use mathematical consequences of the Pauli principle in order to determine the electronic configuration of atoms (The short introduction to many-electron systems is given here).
We have already found out that there are values l = 0,1,2,...,n for
each n value of hydrogen atom. In its turn, for each l value there are
2l + 1 values of ml and for each ml
there are two values of ms (= ±½ ; Spin up and Spin down),
since s = ½.
| Orbital moment l: | 0 | 1 | 2 | 3 | 4 | 5 |
| Symbol: | s | p | d | f | g | h |
| maximum occupied, 2(2l + 1): | 2 | 6 | 10 | 14 | 18 | 22 |
The configurations of first ten elements are given in the diagram below:
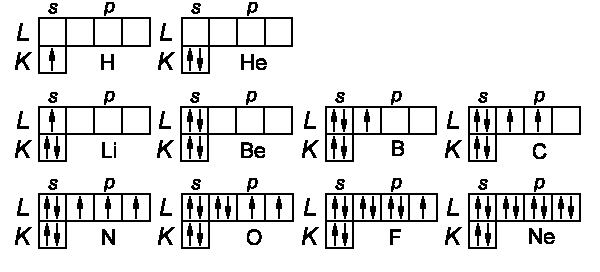
The s-subshell has a block for two electrons having opposite directions of
spins; the p-subshell consists of 3 blocks (ml = −1, 0, +1)
having maximum two spin states, correspondingly. The spin orientation and
J-values for the total kinetic moment can be found according to the following
empirical rules (Friedrich Hund):
Hunds Rules:
One can find periodic table description here: Periodic Table. (a 1st class, a must have by each chemist!)
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.