Now we want to consider the example of Lithiumhydride LiH and apply the LCAO description of molecular orbitals. Ab initio calculations (derived from the self consistent field approach) with a minimal basis (i.e. the Li atom with 1s-, 2s- and 2p-atomic orbitals and Φ1s, Φ2s and Φ2p as wave function and a hydrogen wave function ΦH) produce (neglecting a minor involvement of Φ1s) according to Ransil (1960) the following molecular orbitals
1σ ≈ Φ1s
2σ ≈ 0.323 Φ2s + 0.231 Φ2pz
+ 0.685 ΦH
LiH [(1σ)² (2σ)²]
Thus, in lithium hydride we find an inner shell (1σ)² that is almost identical with the respective atomic orbital, and a bonding 2σ-MO occupied by two electrons. The 2p-AO of lithium needs to be introduced because the 2p-AO is only slightly higher in energy and the condition of similar energies for combining AO is met in the same way as it is with the 2s-AO. Thus, we shall combine all atomic orbitals of a certain energy level and solve the respective secular equation. The solution above has been obtained exactly in this way.
Nevertheless, there is still an opportunity to cling to the simple principle where molecular orbitals emerge from an overlap between two orbitals and that both sides introduce just one orbital. In the following form, the equation allows for this.
2σ = 0.397 (0.813 Φ2s + 0.582 Φ2p) + 0.685 ΦH
This expression is a linear combination of two orbitals, one from lithium is a combination of Φ2s and Φ2p. This combination is called hybrid orbital and the respective approach hybridization. The coefficients within the hybrid wave functionΦhybrid = 0.813 Φ2s + 0.582 Φ2p
have been multiplied in a way that the function is normalized. In general, we require that
∫(a Φ2s + b Φ2p)² dτ = a² + b² = 1
Note that, as the hybrid orbital have the nucleus as a commom centre, "mixed" elements of the kind ∫&Phi2sΦ2pdτ disappear. To characterize such an sp-hybrid, the population, i.e. a² and a², is often introduced in the form of a superscript: s0.661p0.339 (a=0.813, a²=0.661 and b=0.582 b²=0.339). More and more often, we find an notation normalized with respect to s, i.e. in our example sp0.51. Thus the bonding MO of LiH emerges from an overlap between the hydrogene 1s- and the lithium hybrid orbital (h) where the contribution of p is the 0.51-fold of the contribution of s.
2σ = 0.397 Φh + 0.685 ΦH
 |
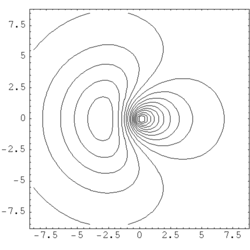 |
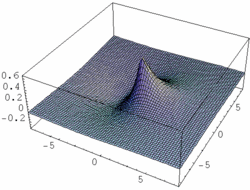
| Fig. 1: Contour map of a hybrid orbital formed by an s-p combination of atomic orbitals. The nodal plane lays beside the nucleus' position which is the origin of the coordinate plane |
Thus, a hybrid orbital is a linear combination of orbitals of one single atom. To an sp hybrid orbital for example, s and p orbitals the atom contribute to the same extent. (see Fig .1). An electron in this state displays 50% s- and 50% p-character. In our example of LiH, the weight of the 2s function was somehow higher than that of the 2p. It was 0.661:0.339 ≈ 1.95:1 instead of 1:1. An sp³-hybrid displays 25% s- and 75% p-character (i.e. s- and p-orbitals contribute with 1 : 3 to the hybrid). Likewise, an sp³d² hybrid is an orbital to which s-, p- und d-functions contribute in the relation of 1:3:2. In general, N atomic orbitals yield N hybrid orbitals orthogonal to each other. There are
The shape of hybrid orbitals is recieved by constructive and destructive interference of the involved atomic orbitals. The large lobes of sp hybrid orbitals formed by one s and one p orbital point in opposite directions. The higher the weight of the p component, the smaller the angle between the orbitals of this type. The four sp³ hybrid orbitals establish the directions found in the tetrahedron, the six sp³d² orbitals in the directions found in the octahedron. The construction of hybrid orbitals is done in a way that the orbitals point in the direction of molecular bonds (respectively in the direction of a non-bonding orbital). As any of the mentioned hybrid orbitals display partial s characteristics, none of them shows a nodal plane in the centre of the atom. The nodal planes typical for p- and d- orbital are shifted to an eccentric position (Fig. 1). The following table contains a list of the most important types of hybridization and the spatial distribution of the respective hybrid orbitals.
| Table 1: Some typical hybridizations | ||
| coordination number | shape | hybridization |
|
|
linear
bent |
sp, pd, sd
sd |
|
|
trigonal planar
unsymmetrical planar trigonal pyramidal |
sp², p²d
spd pd² |
|
|
tetrahedral
irregular tetrahedral quadratic planar |
sp³, sd³
spd², p³d, pd³ p²d², sp²d |
|
|
trigonal bipyramidal
tetragonal pyramidal pentagonal planar |
sp³d, spd³
sp²d², sd4, pd4, p³d² p²d³ |
|
|
octahedral
trigonal prismatic trigonal antiprismatic |
sp³d²
spd4, pd5 p³d³ |
Originally, hybrid orbitals have been introduced in the valence bond theory where all bonds are described as overlap of orbitals of two neighbouring atoms. In the tetrahedral molecule CH4, the bonds C-H are explained by an overlap of the 1s orbitals of four H atoms with four sp³ hybrid orbitals of the carbon atom.
In a correct description of the trigonal pyramidal molecule NH3, one of the four hybrid orbitals should differ from the three others. With 107°, the angle between HNH is smaller than the tetrahedral angle. An explanation for this finding is that the ratio of p in the hybrid orbitals establishing the bonds N-H is higher than in a regular sp³ hybrid. For known angles, the following formula allows to calculate precisely the ratio of p in an spλ hybrid orbital
| cos φ = -1/λ | 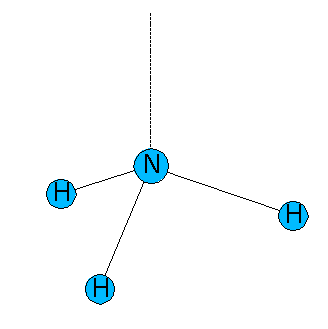 |
Here, φ is the angle between the NH-bonds. For NH3 we recieve λ = 3.42. For known angles, the equivalent hybrid orbitals h = as + bp can be characterized as the following equations yield the values for a and b.
| a² = cos φ/cos φ −1 | b² = 1 - a² |
| a² = 0.226 | b² = 0.774 |
The hybridization of other hybrid orbitals are as well mathematically linked to the angle φ. In the case of NH3, the orbital h' that lays within the three-fold axis of symmetry and to whom we ascribe the non-bonding pair of electrons, emerges as well from combining an s- and p orbitals. As the three hybrid orbitals h display a higher ratio of p (3.42 > 3 in the regular tetrahedron), we expect a minor ratio of p in this fourth hybrid orbital h'.
| h' = a's + b'p' | |
| a' 2 = 1 + cos φ/1 − cos φ | |
| a' 2 = 0.71/1.29 = 0.547 | ⇒ b2 = 0.453 |
| 0.453 : 0.547 = 0.83 | ⇒ sp0.83 - hybrid orbital |
As the hybrid orbitals of the NH bonds display a higher ratio of p, the nonbonding pair of electrons should occupy an orbital with a higher ratio of s.
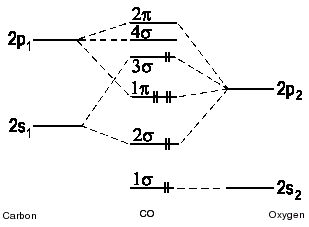 |
| Fig. 2a: A correlation diagram the molecule carbonmonooxide CO without hybridization. The highest occupied molecular orbital (3σ) is antibonding. (only valence electrons shown) |
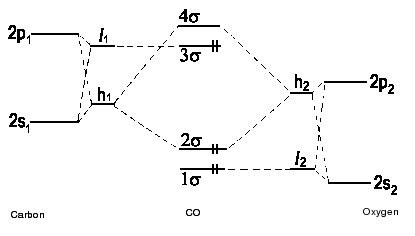 |
| Fig. 2b: A correlation diagram for carbonmonooxide CO with hybridization. Due to the pronounced difference in energy between the bonding and the antibonding σ molecular orbitals, the nonbonding pair of electrons is found in the 3σ- molecular orbital. This is in accordance with experimental findings. (To keep the diagram concise, the π orbitals have been omitted.) |
The orthogonal partner of
h1 = a 2s + b 2p
is
l1 = b 2s − a 2p.
Carbonmonooxide is able to be bound by transition metal ions, a finding that is easily to be explained by the lone pair of electrons on the carbon side of the molecule that interacts the metal cation. The anion CN− and the cation NO+ are isoelectronic to carbonmonooxide. As well, they are found in complex compounds with transition metal elements and bonding in these diatomic species is explained by an analogous electron configuration.
The previous considerations should underline the importance of hybridization in a qualitative theory of chemical bonding. In advance, this concept provides the most important results of a complete wave mechanical calculation: Pairing of orbitals of similar energy and suitable geometry.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.