Selection rules for pure rotational
spectra![]()
A molecule must have a transitional dipole moment that is in resonance with an electromagnetic
field for rotational spectroscopy to be used. Polar molecules have a dipole moment. A transitional dipole
moment not equal to zero is possible.
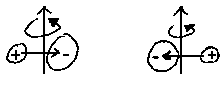 |
| z. B. HCl |
| symmetric stretch mode | 
|
|
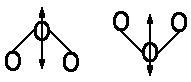 |
bending mode | 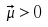 |
| antisymmetric stretch mode | 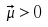
|
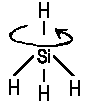 |
| With high rotational speed, an originally spherical symmetry of a molecule is distorted. |
In contrast, no rotational spectra are displayed by homonuclear diatomics; the same is true for spherical tops. Nevertheless, certain states of a such molecules allow unexpected interactions with the electromagnetic field; i.e.
| ΔJ = ▒ 1 | ΔMJ = 0, ▒ 1 |
For a symmetric top, an existing dipole moment is always parallel to the
molecule's axis. Thus, with respect to this axis, no changes of the rotational
state occur.
| ΔK = 0 |
For transitions J + 1 ← J, an equation of the following kind rules the wavenumbers of absorbances to occur.
ν = B(J + 1)(J + 2) - BJ(J +
1)
ν = 2B(J + 1)
with J = 0, 1, 2...
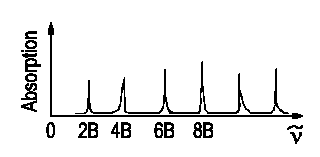 |
The distance between two lines is constant. Δν = ν(J) −ν(J−1) = 2B. We are then able to determine the bond's length r as I = Ár2. |
For high rotational speeds and centrifugal forces that stretch a molecule, a more accurate equation for ν is
| ν = B(J+1)(J+2) - D(J+1)2(J+2)2 - BJ(J+1) + DJ2(J+1)2 |
| ν = 2B(J + 1) - 4D(J + 1)3 |
i.e., the distance between the lines
| Δν = ν(J) - ν (J − 1) |
| Δν = 2B - 4D(3J2+ 9J + 7) |
decreases with J. Thus, the centrifugal constant D for diatomic molecules is in connection with the wavenumber νS that corresponds with the molecule's vibration.
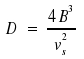
Conversely, D provides information on νs. Of course, the intensity
of an absorption is dependent on the transitional dipole moment and on the
occupancy of the initial and the final state.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.