Examples: CH4, CCl4, SF6
For the case of I = IA = IB = IC the rotational energy is given by:
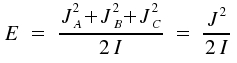
We obtain the respective quantum mechanical equation if we shift to the operators H and J:
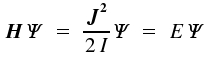
The eigenvalues to J² are already
known: J²⋅Ψ =
J(J+1) h²⋅Ψ with J =
0, 1, 2, ...
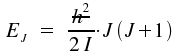
It has become common practice to write this equation in a form with the
rotational constant B which has the unit of the wavenumber,
cm-1. The product term hcB equals the fraction
h2/2I.
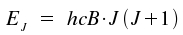
With c denoting the speed of light, the transformation between the moment of inertia I and rotational constant B is
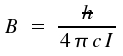
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.