Molecular Symmetry
![]()
As symmetry is fundamental for a plenty of chemical and physical properties of atoms, ions and molecules, this aspect gets more and more attention for anyone who tries to get a deeper understanding for the properties and the reactions of chemical compounds.
E.g., a prerequisite for piezoelectricity is that a crystal displays a certain symmetry. The phenomenon is fundamental for the use of quartz crystals in clocks and the synchronization of electronic devices. On the other hand, a phenomenon like optical activity is associated with the absence of certain symmetry elements.
The correspondence between symmetry and many chemical and physical properties is based on the fact that all wavefunctions - without regard of what the describe mathematically, the distribution of electrons, vibrations of a molecule, nuclear resonance - have to obey some conditions which, in turn, are based on the symmetry of the framework of nuclei.
Discussions on symmetry are of enormous importance in modern chemistry because problems in symmetry can be solved with mathematical methods. They are taken from some branch of linear algebra, the group theory. In contrast to difficult algebraic approaches necessary when establishing important theorems, the tools employed in group theory are simple but surprising fruitful with respect to the results. Considerations of symmetry and solutions based on group theory were important for the proceedings in quantum chemistry and spectroscopy and help nowadays a lot to understand the dynamics of concerted polycentric reactions.
Another useful application of molecular symmetry and the corresponding nomenclature lies in an exact description of structures. Symbols introduced deliver unambigous structural information and are more precise than long texts. For example, the symbol D4h states that the ion [Ni (CN)4]2-
As the use of symmetry symbols spreads more and more in literature, it is indispensable to become familiar with the basics of symmetry, simply to comprehend what is published. We will proceed with a general consideration of this topic and continue with the rules applied in molecular symmetry.
Symmetry and Conservation Laws
Symmetry and properties
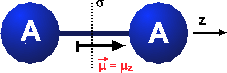
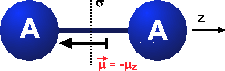
Symmetry in chemistry
Example: Geometric symmetry of objects in a three-dimensional space.
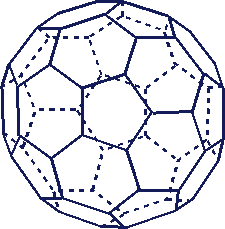
Only four vibrational frequencies have been observed although 3N-6 = 3·60-6 = 174 degrees of freedom exist. Why?
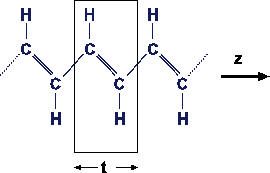
Therefore, the electronic wavefunctions are so-called Bloch functions:
eikx is a planar wave and un(x)
= un(x+t) is a function periodic in space
Symmetry reduces the effort in describing molecules and, in turn, simplifies mathematical treatment of processes. Therefore, group theory is indispensible in theoretical chemistry and became a celebrated tool as it provided approaches to a list of problems
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.