Reflection σ
![]()
A plane that cuts through a molecule in a way that images of all the molecule's features beyond the plane seem to produce an identical molecule is a mirror plane or a plane of reflection. The corresponding operation as well as the plane as symmetry element are denoted with the greek letter σ.
A twofold reflection on one plane produces the operation of identity: σ2 ≡ E. Any planar molecule has at least one mirror plane.
| Representative: ONCl | ||
| Movie, 639kB | ||
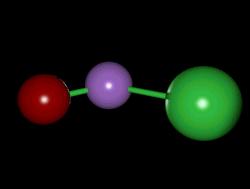
|
||
The orientation of a mirror plane relative to the molecule's main axis is indicated by a subscript. σh indicates a plane which is perpendicular to this axis or horizontal, whereas σv is the symbol for vertical mirror planes containing the main axis. If such a plane bisects the angle between a pair of rotational axis C2, we have a diagonal mirror plane σd.
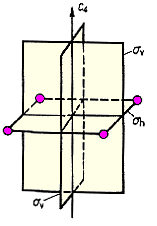
|
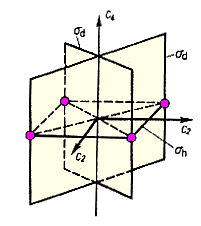 |
| Fig. 1: Three types of mirror planes: σv, σh, σd | |
| Representative: molecule H2O featuring two vertical mirror planes σv | |
| Movie, 467kB | Movie, 502kB |
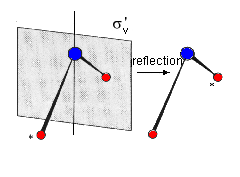 |
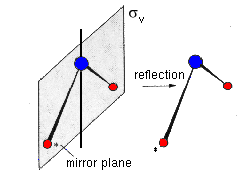 |
| Fig. 2: In the water molecule, the two planes of reflection intersect in axis C2. | |
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.