Like with diatomic molecules, a consideration of the symmetry of the molecule and the symmetry of involved atomic orbitals yields valuable information on the molecular orbitals. As an approximation to molecular orbitals, we will deal with linear combinations of atomic orbitals. Finding the molecular orbitals is facilitated by introducing the aspect of symmetry. Therefore, we establish "symmetry adapted linear combinations" of atomic orbitals, the so-called symmetry orbitals.
Taking the water molecule as an example, we will illustrate this method. We assume a bent structure with identical distances H-O (questions on geometry are answered by the rules of Walsh). The molecule falls into point group C2v. The molecular orbitals of H2O need to show this symmetry and the symmetry operations for C2v affect these orbitals in the same way the respective irreducible representations are affected. For the mentioned point group, all irreducible representations or symmetry species are one-dimensional; thus the values +1 and −1 in the character table for C2v indicate whether an MO behaves symmetric or antisymmetric with respect to
an operation.
|
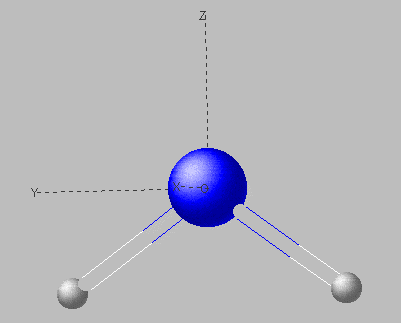 |
An MO of symmetry a2 (we use minuscules letters for single electron states, i.e. orbitals, and majuscules for the total electronic states of molecules) is therefore symmetric with respect to identity E and a rotation on a two-fold axis C2 (If there is a distinct axis, it is taken as axis z). In contrast, it is antisymmetric with respect to a reflection on the planes xz and yz. None of the four symmetry operations affects the framework of the molecule itself.
We would be free to choose an orientation with the two hydrogen atoms in the plane xz or in plane yz normally, but, by convention, the second possibility is preferred and both hydrogen atoms lie within the plane yz and axis z bisects the angle H-O-H.
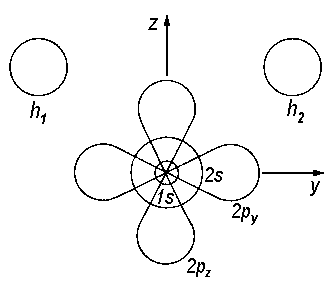 |
| Fig.1: Atomic orbitals of the H2O molecule. The orbital px is perpendicular to the plane yz. |
With respect to point group C2v, this set of orbitals is already symmetry adapted as we will demonstrate for the atomic orbital px. Applying any of the symmetry operations of the group to the orbital's wave function, we ask whether the sign of the wave function remains constant or changes.
Not surprisingly, identity E leaves the wave function unaffected, therefore 1 is the value noted down for px.
| E | C2 | σv | σ'v |
| 1 |
Next we imagine a rotation of 180° about the principle axis and check whether there is a change of sign for the respective wave function. As this is the case, the entry below the symmetry operation C2 px is -1.
| E | C2 | sv | σ'v |
| 1 | -1 |
If the atomic orbital is submitted to reflection on the plane xz, we find the wave function unaltered; therefore the entry below σv(xz) is 1.
| E | C2 | σv(xz) | σ'v |
| 1 | -1 | 1 |
If the atomic orbital is submitted to reflection on the plane yz, we obtain the wave function with reversed sign, therefore the entry below σv(yz) is −1.
| E | C2 | sv | σv'(yz) |
| 1 | -1 | 1 | -1 |
If we compare the values recieved with the entries in the character table of point group C2v, we recognize it to be identical with one set of characters in a row of the table. This correspondence is fundamental for the classification of orbitals to a certain symmetry species. In our example, b1 is obtained for px. In this way, atomic orbitals and the irreducible representations of C2v are linked.
| atomic orbital | s | px | py | pz |
| irreducible representation | a1 | b1 | b2 | a1 |
In contrast to the orbitals of the oxygen atom, the AOs h1 and h2 are not symmetry adapted as C2 or σ(xz) convert h1 in h2 and vice versa. But it is easy to recognize that linear combinations like
| hs = 1/√2 (h1 + h2) |
| ha = 1/√2 (h1 − h2) |
are symmetry adapted. To find the correspondence between the obtained orbital and the symmetry species, we apply the operations. The result is shown in the table below.
| atomic orbital | hs | ha |
| irreducible representation | a1 | b2 |
Using the six symmetry-AOs s, px py, pz,hs and ha, an equal number of MOs can be formed. Note that identical symmetry is a precondition for such combinations.
As there is only one atomic orbital of symmetry species b1, it is not introduced in any linear combination and therefore we regarded px as a molecular orbital of H2O as well. Three AOs are of symmetry species a1. Therefore s, pz and hs are combined to yield three molecular orbitals. Finally, py and ha belong to b2 and are thus combined to yield two molecular orbitals
| 1a1 = c11s + c12pz + c13hs |
| 2a1 = c21s + c22pz + c23hs |
| 3a1 = c31s + c32pz + c33hs |
| 1b1 = px |
| 1b2 = c44py + c45ha |
| 2b2 = c54py + c55ha |
The coefficients cik remain to be determined. It is obvious that, for the two b2 orbitals, these coefficients will have the same sign (we denote this combination with 1b2) and in the other, they will have opposite signs as orthogonality is imposed on the resulting molecular orbitals. Fig. 2 shows these MOs schematically with hatched areas for positive values and blank areas for negative values of the function.
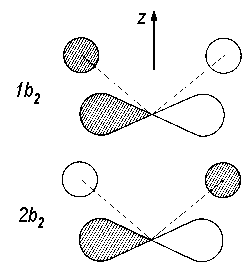 |
| Fig. 2:
Representation of the orbitals 1b2 and the 2b2 in H2O. |
In a situation where several orbitals of the involved atoms fall in one symmetry species (like a1 in our example), each pair of atomic orbitals A or Bn usually yields one bonding and one antibonding linear combination. The remaining atom orbitals become non-bonding MOs.
| bonding: | 1a1 | 1b2 |
| non bonding: | 2a1 | 1b1 |
| antibonding: | 3a1 | 2b2 |
We have derived the electronic configuration of the ground state of the water molecule without introducing the aspect of energy. The sequence of MOs in orbital energy is dependent on
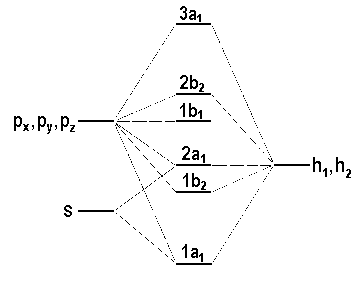 |
| Fig. 3: Qualitative representation of the energy of the valence molecular orbitals in the molecule H2O. |
If, instead of eight electrons, we had twelve to be distributed over the six orbitals, the number of fully occupied bonding and antibonding orbitals would be equal and no stable molecule could exist. For the stability of a molecule it is normally required that the number of doubly occupied bonding orbitals is equal or higher to the number of doubly occupied antibonding MOs. The difference between these numbers is the number of bonds in the molecule.
An additional important aspect in our context is to consider whether there is at all the possibility to form covalent bonds. If the hydrogen orbitals h1 and h2 were of substantially higher energy, we would have linear combinations of approximately this kind
| 1a1 ≈ c11s + c12pz |
| 1b2 ≈ py |
I.e. the electrons were located in the sphere of the oxygen atom and would not contribute to bonding. If we now fill the molecular orbitals 1a1, 1b2, 2a1 and 1b1, we would recieve the oxygen anion O2− and the hydrogen atoms left as protons. But, due to electronic repulsion, there is a limit for the transfer of electrons. This aspect is not revealed by the scheme of MOs. For compounds ABn with strongly asymetric distribution of charges, this scheme could lead to wrong conclusions.
In principle, to understand the electronic configuration of H2O and other molecules of point group
| Kimball table for molecules AB2 of symmetry C2v
for atoms B in the plane yz |
|||||
| Atom | C2v | a1 | a2 | b1 | b2 |
| A | s
p d |
1
1 2 |
0
0 1 |
0
1 1 |
0
1 1 |
| B2 | σ
π |
1
1 |
0
1 |
0
1 |
1
1 |
The table predicts the way atomic orbitals split and transform to irreducible representations of C2v. For atom A, the s-orbital becomes a1, the three p-orbitals become a1, b1 and b2, the five d-orbitals transform to two a1-orbitals, one a2, one b1 and one b2-orbital. The entries for B2 display the kind of irreducible representations emerging from σ and π-AOs respectively. The names σ and π are used with reference to the connecting lines between atoms A and B. Orbitals with rotational symmetry about this axis are classified as σ and orbitals with a nodal plane containing this axis as π. For example,
Applying the Kimball table to the water molecule, we take the s, p and σ states and state that
Often, antibonding molecular orbitals are labelled by an asterisk.
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.