



Next: Selection Rules for Electronic
Up: Molecular Spectroscopy
Previous: Vibrational and Vibrational-Rotational Spectra
Contents
In the Born-Oppenheimer approximation the energy of a molecule can be presented as sum of
electronic energy 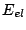 , vibrational energy
, vibrational energy 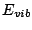 and rotational energy
and rotational energy 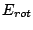 energy.
energy.
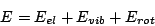 |
(65) |
As a zero-th approximation, we describe the energy 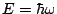 of a diatomic molecule as a
sum of electronic, vibrational and rotational energies
of a diatomic molecule as a
sum of electronic, vibrational and rotational energies
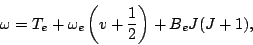 |
(66) |
where 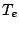 is the minimum of the potential curve. Each of the three terms in eq. (66)
may be different for the lower and higher energy states and may be changes during an optical
transition. The total transition frequency can be written as
is the minimum of the potential curve. Each of the three terms in eq. (66)
may be different for the lower and higher energy states and may be changes during an optical
transition. The total transition frequency can be written as
where the lower energy state is denoted by a double prime (''), while the higher energy state is
denoted by prime (').
Note, that the energy difference corresponding to the excitation of electrons
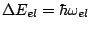 in this approximation is much larger that the energy difference corresponding
to the molecular vibration
in this approximation is much larger that the energy difference corresponding
to the molecular vibration
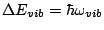 which is large that the energy
difference corresponding to the molecular rotations
which is large that the energy
difference corresponding to the molecular rotations
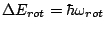 :
:
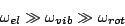 |
(68) |
Subsections




Next: Selection Rules for Electronic
Up: Molecular Spectroscopy
Previous: Vibrational and Vibrational-Rotational Spectra
Contents
Markus Hiereth
2005-01-20
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.
![]() of a diatomic molecule as a
sum of electronic, vibrational and rotational energies
of a diatomic molecule as a
sum of electronic, vibrational and rotational energies
![]() in this approximation is much larger that the energy difference corresponding
to the molecular vibration
in this approximation is much larger that the energy difference corresponding
to the molecular vibration
![]() which is large that the energy
difference corresponding to the molecular rotations
which is large that the energy
difference corresponding to the molecular rotations
![]() :
: