Infrared Absorption Spectroscopy (IR)
Introduction
Infrared (IR) absorption spectroscopy is the measurement of the wavelength
and intensity of the absorption of mid-infrared
light by a sample. Mid-infrared light (2.5 -
50 µm, 4000 - 200 cm-1) is energetic enough to excite
molecular vibrations to higher energy levels. The wavelength of many IR
absorption bands are characteristic of specific types of chemical bonds,
and IR spectroscopy finds its greatest utility for qualitative analysis
of organic and organometallic molecules. IR spectroscopy is used to confirm
the identify of a particular compound and as a tool to help determine the
structure of a newly synthesized molecule (with NMR spectroscopy
and mass spectrometry).
Mechanism of IR absorption
The transition moment for infrared absorption is:
R = < Xi | µ | Xj dt >
where Xi and Xj are the initial and final states,
respectively, and µ is the electric dipole moment operator:
µ = µo + (r-re)(dµ/dr)
+ ... higher terms.
µo is the permanent dipole moment, which is a constant,
and since < Xi | Xj > = 0, R simplifies to:
R = < Xi | (r-re)(dµ/dr) | Xj
>
The result is that there must be a change in dipole moment during the
vibration for a molecule to absorb infrared radiation.
Examples of infrared active and inactive absorption bands in CO2
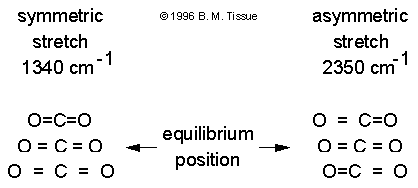
There is no change in dipole moment during the symmetric stretch vibration
and the 1340 cm-1 band is not observed in the infrared absorption
spectrum (the symmetric stretch is called infrared inactive). There is
a change in dipole moment during the asymmetric stretch and the 2350 cm-1
band does absorb infrared radiation (the asymmetric stretch in infrared
active). A related vibrational spectroscopic method is Raman
spectroscopy, which has a different mechanism and therefore provides
complementary information to infrared absorption.
Instrumentation
Instrumentation for dispersive and Fourier-transform IR absorption instruments
are described in a separate document.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.

