For two atomic homonuclear molecules like H2, O2, N2 etc, the LCAO expansion represents an especially simple approach. The progress made with the ionic molecule H2+ is as well valuable for other homonuclear species. We will treat theses molecules in the same way. Of course, we will face some additional points, especially for bonds established by more than one electron. Analogous to atomic orbitals, we introduce molecular orbitals of different energy. The molecule's electrons are distributed among them in a way that the orbitals with the lowest energies are occupied first and the Pauli principle is accomplished. For a first approximation, we regard these orbitals as linear combinations of atomic orbitals. Taking the two 1s orbitals of the hydrogen atom, we obtain the two MOs that already appeared in the previous chapter that dealt with H2+:
In order to have a concise presentation, the various coeffients for normalization are omitted in both equations. Moreover, greec minuscules σ (analogous to s for the atoms) are introduced to represent the MOs. A consideration of the indices follows below. Note that αA and αB in the secular equations are identical for homonuclear molecules: αA = αB = α. Therefore, both wave functions are introduced with the same weight but maybe with opposite signs.
Thus, in cases where the overlap integral S is, compared with 1, small, an originally degenerated pair of energy values α splits symmetrically into two values above and below the original energy level.
The level of reduced energy is called the bonding MO as it is below the energy level of the separated atoms. The level of increased energy is above the energy level of the separated atoms and is called antibonding MO. Horizontal lines in the following figure represent the two atomic and the two molecular orbitals and the respective energy of an electron within this orbital.
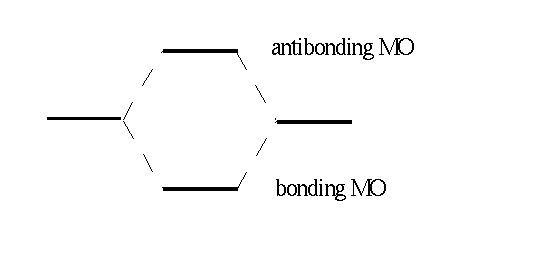
Now we allocate the MOs, beginning with the orbital lowest in energy and having the Pauli principle in mind. For H2 we obtain wave funcion σg(1) for one electron and σg(2) for the other. For the total wave function our approach yields:
The first two terms indicate that both electrons are located near different nuclei (for H: protons) whereas the last two terms describe two electrons located around one single nucleus (A resp. B). There is another approach by Heitler-London that just ignores the last two terms argueing that the repulsion between the two electrons will make this situation quite improbable (i.e. there is an interdependence between the electrons). But occasionally, this happens. Therefore, MO and Heitler-Londer's are two extreme approaches and a superior description is to be found between the two, e.g. in the so-called ionic approach with c as variation constant:
Ψges = σA(1)ΦB(2)+ΦA(2)ΦB(1) + c[ΦA(1)ΦA(2)+ΦB(1)ΦB(2)]
An overview to possible and impossible combinations of atomic orbitals of the types s, p and d is given in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| #Possible combinations for py and dyz are obtained by exchanging x and y in any of the two lines | ||
The figure below depicts the additive and subtractive combination of p-orbitals to give two MOs. By convention, direction z is in line with the axis between both nuclei. (Click here for further examples of MOs and for the specific cases of 1s+1s and 2s+2s. Subtracting one pz orbital from another yields the bonding LCAO σg, whereas the linear combination obtained by adding the two pz orbitals leads to the antibonding orbital σu. From combinations of px or py orbitals π-orbitals emerge; thus, these orbitals are doubly degenerated. An additive combination of px or py AOs produces a marked overlap and therefore a bonding MO (πu). In contrast, subtractive combination gives rise to antibonding MOs (πg).
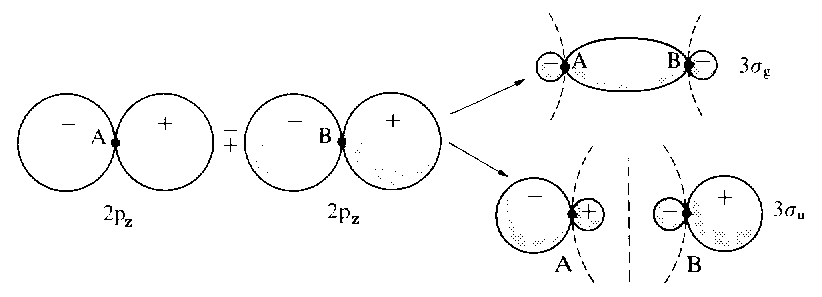 |
| From combinations of two
2pz atomic orbitals one bonding and one antibonding molecule orbital emerge, labelled as |
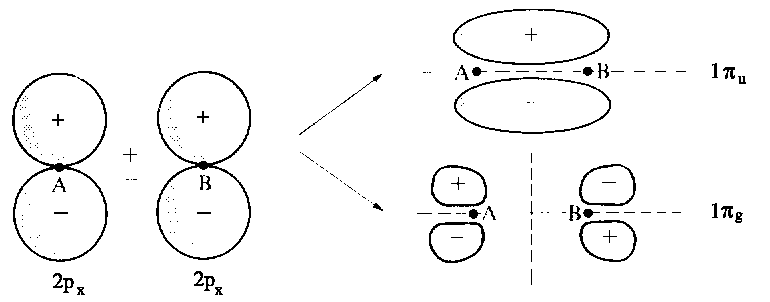 |
| Combination of two 2pxatomic orbitals gives as well rise to one bonding and one antibonding molecular orbital, labelled as |
The symbols for classification of molecular orbitals are similar to those used for atomic orbitals. The symbols s, p, d, ... for atoms correspond to σ, π and δ and refer to the angular momentum of the electron with respect to the axis between the two nuclei and to symmetry of the molecule orbital.
With the numbers in front of the symmetry symbol, molecular orbitals of one symmetry are classified with respect to their energy. The higher the number is, the higher is the energy of an electron within this state.
The sequence of MO energies implies structural properties of molecules in a similar way the energy levels of AOs are used to explain the properties of atoms. The currently available data support the following succession of molecular orbitals:
|
|
For atoms of the second row of the periodic table forming two atomic molecules, the σg-MO is always below the πu-MO. Note that π-MOs appear doubly degenerated. 1πu is therefore used as an abbreviation for the pair of orbitals 1πxu and 1πyu that evidently come from the px- and the py atomic orbital.
Now we are able to use the succession of MOs to describe homonuclear two atomic molecules. The electrons are distrubuted among the orbitals according energy and the Pauli principle. Due to degeneracy, up to four electrons are allocated to the π level. We already know that the two electrons of the hydrogen molecule H2 occupy the bonding MO, thus the molecules electronic configuration is (1σg)2. If we continue our consideration with He2+, we would expect the ground state configuration (1σg)2(1σu) and He2 would have (1σg)2(1σu)2. In fact, the molecule He2 is found to be instable in its ground state whereas it is stable in configurations with one electron in a molecular orbital of higher energy, e.g. in a configuration like (1σg)2(1σu)(2σg)).
The instability of He2 is one example of the general rule that occupation of both the bonding and the respective antibonding molecular orbital does not yield a chemical bond; in fact the antibond wins slightly over the bond because the splitting of the two involved atomic orbital energy levels is not perfectly symmetric.
The MO description tends to ignore the inner shell electrons and descriptions of electronic configurations just care for the former valence electrons. For example Li2 with two valence electrons is described with the inner shell electrons as
Li2 [ (1σg)2(1σu)2(2σg)2]
or, shortly by introducing capital letters for complete shells
Li2 [ KK(2σg)2 ]
In a similar manner, we describe the electronic configuration of fluorine F2
F2 [ KK(2σg)2(2σu)2(1πu)4(3σg)2(1πg)4 ],
where (1πu)4 denotes the degenerated pair of (1πxu)2(1πyu)2. 2σg and 2σu as well as 1πu and 1πg are pairs of bonding and antibonding MOs. Therefore, of the 14 valence electrons only the pair of electrons in (3σg)2 are regarded as bonding and this corresponds to the finding of a single bond of σ-type in the molecule.
The total configuration (term symbols) is derived from the electronic configuration. Clicking will deliver details of the procedure and a summary for the term symbol notation. These term symbols consist of greek capital letters Σ, Π, Δ, ... (indicating the projection of the total angular momentum respective the axis between the two nuclei), the subscript g or u (for molecules with a centre of symmetry) and a preceding superscript (indicating the spinmultiplicity 2S+1 for the total spin S). The sign superscript for Σ states indicates the effect of reflecting the wave function on an arbitrary plane that contains both nuclei.
Examples:
2Πu total spin = 1/2; total angular momentum = 1; inversion changes the wave function's sign
3Σg-
total spin = 1; total angular momentum = 0; inversion does not change the wave function's sign
| Electronic configuration for two atomic homonuclear molecules in their ground state | ||||||
| electronic configuration* | Number of
valence electrons
bond. - antibond. |
bonds |
Bonding
Energy in eV |
Term symbol | ||
| H2+ | 1σg | 1 | 0 | ½ | 2.793 | 2Σg+ |
| H2 | 1σg2 | 2 | 0 | 1 | 4.748 | 1Σg+ |
| He2+ | 1σg2 1σu | 2 | 1 | ½ | 2.470 | 2Σu+ |
| He2 | 1σg2 1σu2 (=KK) | 2 | 2 | 0 | 0.001 | 1Σg+ |
| Li2+ | KK 2σg | 1 | 0 | ½ | 1.46 | 2Σg+ |
| Li2 | KK 2σg2 | 2 | 0 | 1 | 1.068 | 1Σg+ |
| Be2 | KK 2σg2 2σu2 | 2 | 2 | 0 | 0.096# | 1Σg |
| B2 | KK 2σg2 2σu2 1πu2 | 4 | 2 | 1 | 3.08 | 3Σg− |
| C2+ | KK 2σg2 2σu2 1πu3 | 5 | 2 | 1½ | 5.40 | 2Πu |
| C2 | KK 2σg2 2σu2 1πu4 | 6 | 2 | 2 | 6.32 | 1Σg+ |
| N2+ | KK 2σg2 2σu2 3σg 1πu4 | 7 | 2 | 2½ | 6.478 | 2Σg+ |
| N2 | KK 2σg2 2σu2 3σg2 1πu4 | 8 | 2 | 3 | 7.519 | 1Σg+ |
| O2+ | KK 2σg2 2σu2 3σg2 1πu4 1πg | 8 | 3 | 2½ | 6.781 | 2Πg |
| O2 | KK 2σg2 2σu2 3σg2 1πu4 1πg2 | 8 | 4 | 2 | 5.214 | 3Σg− |
| F2+ | KK 2σg2 2σu2 3σg2 1πu4 1πg3 | 8 | 5 | 1½ | 3.405 | 2Πg |
| F2 | KK 2σg2 2σu2 3σg2 1πu4 1πg4 | 8 | 6 | 1 | 1.659 | 1Σg+ |
| * MOs are not always listed according their respective energy level
# calculated by R. Gdanitz (Dec. 1998) |
||||||
First: The notions of single, double and triple bond refer to the number of pairs of electrons with an effective contribution to bonding. σ and π denotes the type of bond. Typical electronic configurations are
single bond σ2, double bond σ2π2, triple bond σ2π4.
For atoms of the first two rows, no other bonds exist. In contrast, for heavier atoms we face additional possibilities. E.g. there are strong bonds between metal atoms with the configuration σ2π4δ2 that would be regarded as quadrupole bond.
Second: The principle of maximal overlap predicts that π atomic orbitals with their lobes orthogonal to the axis between the nuclei will not overlap to the same extent as σ atomic orbitals where the distribution of charge is colinear with the axis. Therefore, π bonds are weaker than σ bonds. In analogy, the repulsion caused by antibonding σ orbitals exceed the attraction due to bonding π MOs.
Third: Hund's rule for the allocation of electrons in degenerated MOs (e.g. πx, πy) applies in the same way as with AOs in atoms. Thus, for molecular oxygen O2, all levels up to 1πu are occupied and electrons with parallel spin in the degenerated 1πg levels cause the triplett ground state (S = 1).
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.