![]()
In atomic spectroscopy, all transitions reflect changes in the configuration of electrons. Molecules are, in addition, subject to rotational and vibrational states. Consequently, their spectra are more complex but they also yield information about the structure of a molecule and the strength of its bonds. Assuming indepent modes of motion, we can split the total energy of a molecule into the components Eel, vibrational Evib and rotational Erot energy.
E = Erot + Evib + Eel
The energies related to changes between these modes differ greatly:
hνrot << hνvib << hνel
| νrot | Transistions between neighbouring rotational levels | far infrared or microwaves |
| νvib | Transitions purely related to vibrational modes | within infrared (around 1000 cm-1) |
| νel | electronic transitions | visible and ultraviolet light |
In general, electronic transitions introduce changes in the rotational and the vibrational mode. Selection rules determine whether or not transitions are allowed. As the electric field of absorbed or emitted light is able to affect the distribution of electrons within a molecule, there is no need for the molecule to display a permanent dipole moment. In contrast, for rotational-vibrations transitions within a defined electronic state, such a dipole moment is required for a transition to take place.
First in this chapter, a mathematical description of the rotational energy levels is delivered. By taking selection rules into account, an analysis of pure rotational spectra is feasible. With quantities of energy which are able to excite the molecule into a vibrational mode, we expect simultaneous excitation of rotational energy levels. For even larger amounts of energies that change the state of an electron, the vibrational and rotational state of a molecule will be affected as well.
The probability for a transition from state n to state m is
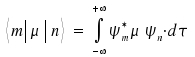
Expression dτ represents volume elements. It is possible to determine this probability for known pairs of wave functions.
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.