In chapter Optical Transitions, the theoretical quantities transition dipol moment µmn, matrix element Rmn, oszillator strength fmn and Einstein's factors Amn and Bmn for spontaneous and induced emission as well as Bnm for the phenomenon of absorption were presented. Now we aim on how to determine these quantities experimentally. First, we consider measurements where emission and absorption are resolved within time, then "ordinary" measurements of absorption. Treating emission like a first-order decay, the reduction of an initial number of excited species within time is
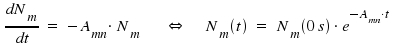
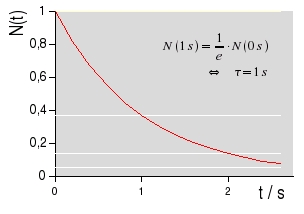 |
Nm(0s) is the initial number of particles within a certain volume and dNm / dt is the number of emitted photons per time. The life time τ of a state is defined as the time needed for a reduction by the factor of 1/e. Introducing τ yields the equation
|
Provided no processes like collisions with other particles or internal conversions occur, a measurment of the life time τ directly yields the transition probability Amn. As the mentioned competitive processes reduce the life time, a higher transition probability is pretended. Having calculated Amn, further theoretical quantities are within reach.
According to Lambert-Beer's law, light of an initial intensity Io passes through a sample with an intensity Itrans when a beam travels along a path with length l where an absorbing substance is found. The concentration N this substance is here defined as the number of particles per volume. σ is introduced as the absorption coefficient for a defined wavelength.
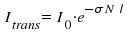
Adequate units are cm2 for σ cm-3 for N and cm for l. Using experimental data, we are now able to determine the absorption coefficient σ.
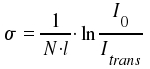
σ is dependent on the frequency ν. Through integration of σ over the frequencies &nu within an absorption peak, we obtain the total intensity for a transition n to m, which is connected with Bnm, the coefficient that reflects an atomic or molecular property.
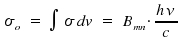
The obtained equation allows to estimate experimental values from an theoretical approach and, vice versa, to determine a transition coefficient Bnm by using experimental data. The above mentioned integration of σ over frequencies leads to the integrated absorption coefficient σo.
In laboratory practice, the extinction ε is the preferred measure to characterize the absorption of light by a substance. It is given in units of We use the quotient Itrans/I0 as a link between the two coefficients
As we introduced concentration N in molecules per volume and the unit of c is moles per volume, Avogadro's number NA = 6.022·1023mol-1 appears.
NA appears as well in the transformation factor between σ and ε. For this factor, we obtain
The conversion factor when using the conventional units dm3mol-1cm-1 for ε and cm2 for σ is 2,62 · 1020. Further conversions between experimental quantities are found here. Due to integration over dν, the unit for frequency, Hertz or s-1, becomes part of the unit of the integrated coefficients σo = ∫σdν resp. ∫εdν. Occasionally, integration is as well done over wavenumbers. Therefore, pay attention to the given units. Finally, an overview on the essential formulas:
All of the quantities are integrated and refer to a center frequency ν. In the next chapter, we will focus on the width of a line, i.e. σ(ν).
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.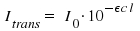
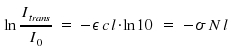
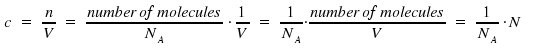
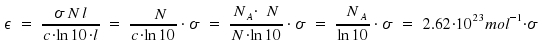
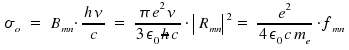
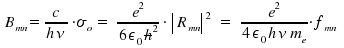
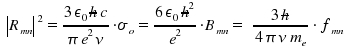
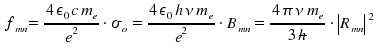
![]()