As well for a treatment of heteronuclear molecules, the qualitative aspects of the LCAO method apply. To get a successful combination of the kind Ψ = cAΦA + cBΦB
Let us apply the conditions for an effective linear combination of atomic orbitals to the hydrogene chloride molecule. The first condition, for which mathematical evidence will be delivered below, forbids combinations between a hydrogene AO and 1s-, 2s- and 2p-orbitals of of chlorine for these AOs (the 3s-orbital might be classified as a borderline case) are of too low energy. In contrast, the atomic orbitals 3px, 3py and 3pz remain as candidates for combinations with the 1s-AO which is the orbital of lowest energy of the hydrogene atom. Taking symmetry into account, the 3pz-AO of chlorine is the orbital that has to be introduced in an LCAO approach that shall describe HCl. As we will learn in one of the following chapters (Hybridization), the 3s-AO of Cl is to some degree involved in this combination, but this does not call the description presented here into question. From these considerations and from the building-up principle, the ground state of the hydrogene chloride molecule is:
(1s)2 (2s)2 (2pz)2 (2px)2 (2py)2 (3s)2 (3px)2 (3py)2 {cAH(1s) + cBCl(3pz)}2
Where the chlorine atomic orbitale are simply denoted as 1s, 2s, ... 3py. Though these orbitals do not contribute to bonding, they appear like MOs of related symmetry, i.e. the s- and pz-orbitals as σ- and the px and py π-molecular orbitals. A σ-state represents the orbital emerging from the {cAH(1s) + cBCl(3pz)}. The energy level of this MO is below the π-orbitals occupied by the (3px)and (3py) electrons of chlorine. With numbers added, the electronic configuration of the molecules is described as:
| (1σ)2 (2σ)2(3σ)2 (1π)4 (4σ)2 (5σ)2(2π)4: 1Σ |
The relation between an orbital of the molecule AB in the LCAO approach and the atomic orbitals, i.e. the components for this approach (ΦA and ΦB) are worth being considered, especially in the case where this molecular orbital is occupied by electrons that can be regarded as valence electrons of the unbound atoms A and B. Assuming a possibility to separate the atoms that form molecule AB, we expect such electrons to appear again in the two atomic orbitals ΦA and ΦB. (We already encountered this aspect when treating the hydrogene cation). Thus, it is allowed to regard these electrons as originating from a ΦA- or ΦB-state, now sharing a state
Let us have a look on the mathematical treatment. For all relevant solutions of the secular equation for a heteronuclear molecule AB
cA(αA−E) +
cB(β − E·S) = 0
cA(β − E·S) +
cB(αB−E) = 0
the determinant becomes zero, i.e. the values for energy E accomplish equation
(αA-E)(αB-E) - (β-ES)2 = 0
To avoid the effort of deriving the exact solution of this equation quadratic in E, we assume that the energies of the bonding MO and the lowest of the two AOs differ only slightly. In case that ΦA is this AO, E ≈ αA−ΔE reflects this assumption without further restrictions to the general applicability. The ΔE represents the shift in energy that corresponds with the bonding effect. Replacing E in the quadratic equation yields
(αA - αA+ ΔE)(αB - αA + ΔE) = (β - (αA - ΔE)S)2
As ΔE << αA, we can write
ΔE·(αB - αA) = (β - αAS)2
and the following expression for the bonding energy is obtainedΔE = [ β - αAS ]2 / [ αB-αA ].
For the energy level for the bonding MO we get:
| Ebonding = αA- [ β - aAS ]² / [ αB - αA ] |
In an analogous way, we derive the energy of the antibonding MO, starting with E ≈ αB-ΔE.
(αA - αB+ ΔE)(αB - αB +ΔE) = (β - (αB - ΔE) S)2
As ΔE << αB, we can write
(αA - αB)·ΔE = (β - αBS)2
and the following expression for repulsion is obtainedΔE = − [ β - αBS ]2 / [ αB-αA ]
For the energy level for the antibonding MO we get:
| Eantibonding = αB+ [ β - aBS ]² / [ αB - αA ] |
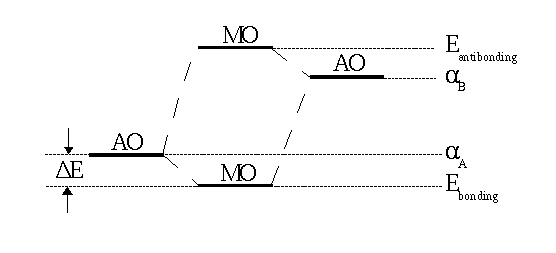 |
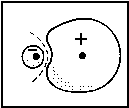
| Fig. 1: In a heteronuclear molecule the bonding MO is closer to the more electronegative atom (A in the case shown here ). In the respective wave function |
The larger the difference between αA and αB, the larger is the denominator in the fraction and, in consequence, the smaller is the splitting of the MO energy levels. These levels are shown in
To obtain the coefficients cA and cB that represent the weight of the single atomic orbitals, we introduce the obtained expression for
cA/cB =
(β - aAS)/ΔE =
- [αB - aA]/[β - αAS] Coefficient cA emerges when Ψbonding is normalized. In analogy, for the antibonding MO we recieve
cB/cA =
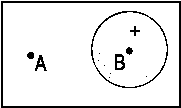
(β - aBS)/[αB-αA] As a threedimensional plot, Fig. 2 illustrates a linear combination with differently weighted atomic wave functions. ΦA is an 2pz-, ΦB is an 1s-orbital. The ratio cB/cA is 3/7.
ψbonding
= cA [ ΦA +
[αAS
- β]/[αB - αA]·ΦB]
ψantibonding
= cA [ ΦA -
[αBS
- β]/[αB - αA]·ΦB]
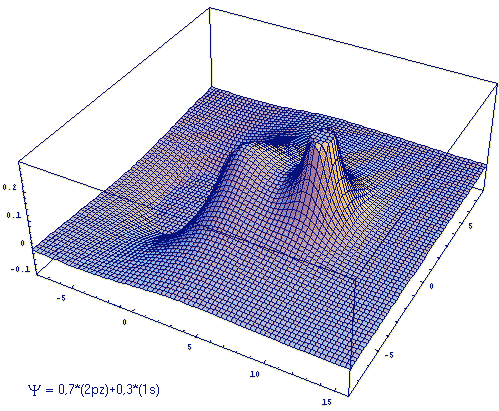
Fig .2: Example for the wave function that emerges from a linear, differently weighted combination of an 2pz and an 1s atomic orbital.
Fig. 3 shows a correlation diagram for a heteronuclear diatomic molecule (comp. homonuclear case). Just σ and π appear as the g-u-classification is only relevant for homonuclear diatomics. Fig. 4 qualitatively shows the distribution of the wave function's amplitude for the single atoms and the orbitals of an hypothetical united atom as the two extremes in this approach. The intermediate case between shall deliver an impression of the conditions in a molecule AB.
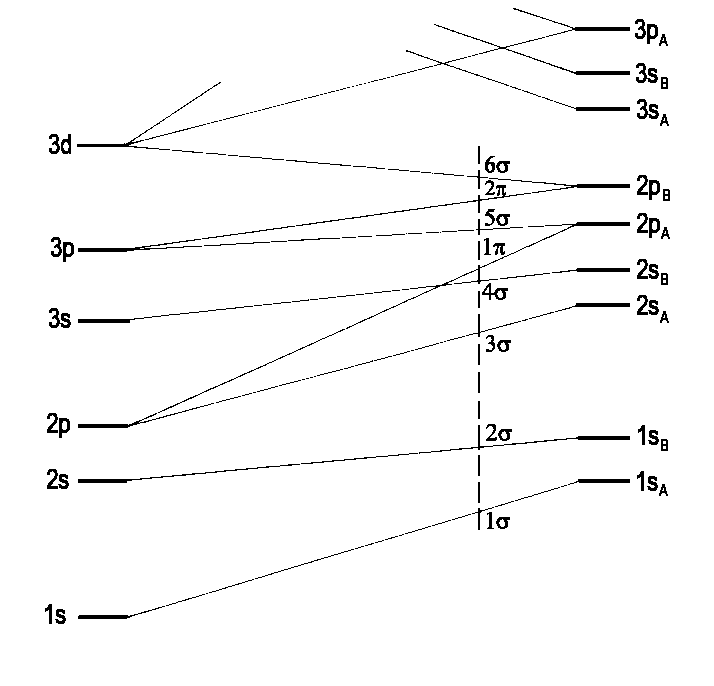 |
| Fig.3: Correlation diagram for a heteronuclear diatomic molekule (compare Fig. 1 Energy Levels in homonuclear molecules). Only σ and π molecular orbitals are of interest. The vertical dashed line represents the sequence of MO energies found in the Molecule NO (according to R.S. Mulliken, Rev. mod. Phys. 4, 1 (1932)). |
| 1s |  |
<=> |  |
<=> |  |
1sA |
| 2s |  |
<=> |  |
<=> |  |
1sB |
| 2px |  |
<=> |  |
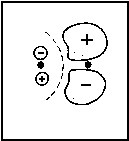
<=> | 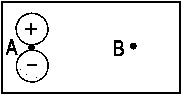 |
2pxA |
| 3px | 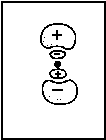 |
<=> |  |
<=> | 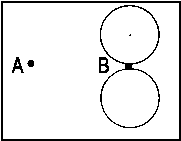 |
2pxB |
| united atom | molecule | single atoms | ||||
| Fig. 4: Examples of the correlation between orbitals of a united atom and the single atoms for a heteronuclear diatomic molecule. The atomic orbitals of the separate atoms on the right yield in one bonding and one antibonding combination one molecular orbital (in the middle) and, in the hypothetical case of an internuclear distance zero, atomic orbital (left). Pay attention to the case of the antibonding combination depicted in the last row. Here, an additional node appears and an AO with an increased quantum number (3px) is introduced. | ||||||
| Electron configuration and term symbols of the lowest states of XH | ||
| XH molecule | ground state | first excited state |
| LiH, BeH+
NaH, MgH+ CuH, ZnH+ |
K(2sσ)2:
1Σ+
KL(3sσ)2: 1Σ+ KLM(4sσ)2: 1Σ+ |
2sσ2pσ:
1Σ+
[3Σ+]
3sσ3pσ: 1Σ+ [3Σ+] 4sσ4pσ: 1Σ+ [3Σ+] |
| MgH, AlH+
CaH HgH |
KL(3sσ)2
3pσ: 2Σ+
KLMsp(4sσ)2 3dσ: 2Σ+ KLMNOspd(6sσ)2 6pσ: 2Σ+ |
(3sσ)2 3pπ:
2Πr
(4sσ)2 3dπ: 2Πr (6sσ)2 6pπ: 2Πr |
| BH, CH+ | K(2sσ)2 (2pσ)2: 1Σ+ | (2sσ)2 2pσ2pπ: 1Π, 3Π |
| CH | K(2sσ)2 (2pσ)2 2pπ: 2Πr | (2sσ)2 2pσ (2pπ)2: [4Σ-], 2Δ, 2Σ+, 2Σ- |
| NH, OH+ | K(2sσ)2 (2pσ)2 (2pπ)2: 3Σ-, 1Δ, 1Σ+ | (2sσ)2 2pσ (2pπ)3: 3Π, 1Π |
| OH | K(2sσ)2 (2pσ)2 (2pπ)3: 2Πi | (2sσ)2 2pσ (2pπ)4 : 2Σ+ |
| HF
HCl |
K(2sσ)2
(2pσ)2 (2pπ)4:
1Σ+
KL(3sσ)2 (3pσ)2 (3pπ)4 : 1Σ+ |
(2sσ)2 (2pσ)2
(2pπ)3 3sσ:
[3Π], [1Π]
(3sσ)2 (3pσ)2 (3pπ)3 4sσ: 3Π, 1Π |
| NiH | KLMsp(3dσ)2 (3dπ)4 (3dδ)3 (4sσ)2: 2Δ | (3dσ)2 (3dπ)4 (3dδ)3 4sσ4pσ: 2Δ, 2Δ, 4Δ |
| For the excited electron configurations the closed atomic shells have not been repeated. The states in brackets have not been observed. Msp means that in the M shell only the groups 3s and 3p are closed, and similarly in other case. | ||
![]()
Auf diesem Webangebot gilt die Datenschutzerklärung der TU Braunschweig mit Ausnahme der Abschnitte VI, VII und VIII.